Abstract
Background:
This study aimed to characterize movement-evoked pain during tendon loading and stretching tasks in individuals with Achilles tendinopathy, and to examine the association between movement-evoked pain with the Achilles tendinopathy type (insertional and midportion), biomechanical, and psychological variables.
Methods:
In this laboratory-based, cross-sectional study, 37 individuals with chronic Achilles tendinopathy participated. Movement-evoked pain intensity (Numeric Rating Scale: 0 to 10) and sagittal-plane ankle biomechanics were collected simultaneously during standing, fast walking, single-leg heel raises, and weight-bearing calf stretch. Description of symptoms, including location of Achilles tendon pain and duration of tendon morning stiffness, as well as pain-related psychological measures, including the Tampa Scale of Kinesiophobia were collected. Linear mixed effects models were built around two paradigms of movement-evoked pain (tendon loading and stretching tasks) with each model anchored with pain at rest.
Findings:
Movement-evoked pain intensity increased as task demand increased in both models. Lower peak dorsiflexion with walking (β=−0.187, 95% CI: −0.305, −0.069), higher fear of movement (β=0.082, 95% CI: 0.018, 0.145), and longer duration of tendon morning stiffness (β=0.183, 95% CI: 0.07, 0.296) were associated with greater pain across tendon loading tasks (R2=0.47). Lower peak dorsiflexion with walking (β=−0.27, 95% CI: −0.41, −0.14), higher dorsiflexion with the calf stretch (β=0.095, 95% CI: 0.02, 0.16), and insertional Achilles tendinopathy (β=−0.93, 95% CI: −1.65, −0.21) were associated with higher pain across tendon stretching tasks (R2=0.53).
Interpretation:
In addition to exercise, the ideal management of Achilles tendinopathy may require adjunct treatments to address the multifactorial aspects of movement-evoked pain.
Keywords: Achilles tendon, tendinopathy, pain, movement-pain
1. Introduction
Clinicians often assess movement-evoked pain (MeP) to quantify the impact of pain on function and disability. Unlike spontaneous or resting pain, MeP is acutely provoked by a specified movement task (Corbett et al., 2019). MeP is generally more severe than resting pain (Fullwood et al., 2021), which limits daily activities (Sluka et al., 2018) and exercise participation (Leemans et al., 2022). Despite this, most pain questionnaires are specific to pain at rest or depend on recall bias to capture the average or maximum pain levels over a period of time, regardless of activity (Corbett et al., 2019). Further, MeP often has multiple measures to capture the temporal aspect of pain changing with movement tasks. These factors have contributed to MeP being an understudied area and warranting further research (Fullwood et al., 2021).
Achilles tendinopathy (AT) is characterized by minimal to no Achilles tendon pain at rest and MeP with tendon loading tasks (de Vos et al., 2021). Tendon loading exercises and stretching tasks are commonly used for clinical assessments and interventions for AT. The optimal starting point when introducing a progressive tendon loading program may depend on the specific subtype of AT present. Progressive tendon loading exercise, considered the standard of care for midportion AT, is frequently performed into end range ankle dorsiflexion (Beyer et al., 2015; de Vos et al., 2021; Rompe et al., 2008, Silbernagel et al., 2007; Stevens and Tan, 2014). In contrast, tendon loading exercise for insertional AT is often conducted in a neutral ankle position, at least at the initiation of care, to minimize the compression of tissues in the insertional region against the posterosuperior aspect of the calcaneus (Chimenti et al., 2017; de Vos et al., 2021; Jonsson et al., 2008). While these different treatment approaches have emerged to manage MeP, little is known about the influence of AT type or other contributing factors to MeP.
In addition to AT type, there are a multitude of factors that can contribute to the development and persistence of AT pain (Franceschi et al., 2014; Magnan et al., 2014). Altered foot and ankle biomechanics, such excessive pronation and eversion, are associated with AT and could aggravate MeP (Chimenti et al., 2016; Dowling et al., 2015; Ogbonmwan et al., 2018; van Der Vlist et al., 2019). However, most research has been exclusive to runners, and little remains known about biomechanics during lower-level activities. Also, pain-related psychological factors, such as fear of movement, are common in individuals with AT and may influence MeP (Chimenti et al., 2021, 2020; Hasani et al., 2021; Sancho et al., 2019; Vallance et al., 2021). Therefore, to better understand MeP as a defining symptom of AT, further research is needed to examine the relative influences of biomechanical and psychological factors on MeP.
The first purpose of this study was to characterize MeP during tendon loading and stretching tasks, relative to pain at rest, in individuals with chronic AT. We hypothesized that pain intensity would be more severe with increasingly demanding tasks for both the tendon loading and stretching progression. The second purpose was to examine the association of MeP with the type of AT, biomechanical, and pain-related psychological variables. We hypothesized that participants with insertional AT would report a similar level of MeP during tendon loading activities (fast walking, heel raises) and more severe MeP during tendon stretching (standing, calf stretch). We further hypothesized that lower peak ankle dorsiflexion, lower ankle power, and higher fear of movement would be associated with higher MeP.
2. Methods
2.1. Design
This is a secondary sub-group analysis from a larger randomized controlled trial, Tendinopathy Education on the Achilles (TEAch) (Chimenti et al., 2023). The study was approved by the University of Iowa Institutional Review Board and prospectively registered at Clinicaltrials.gov (NCT04059146) and Open Science Framework (https://osf.io, JF2XU). All participants provided informed consent prior to participating and data were securely stored in an electronic data management system (REDCap).
2.2. Participants and setting
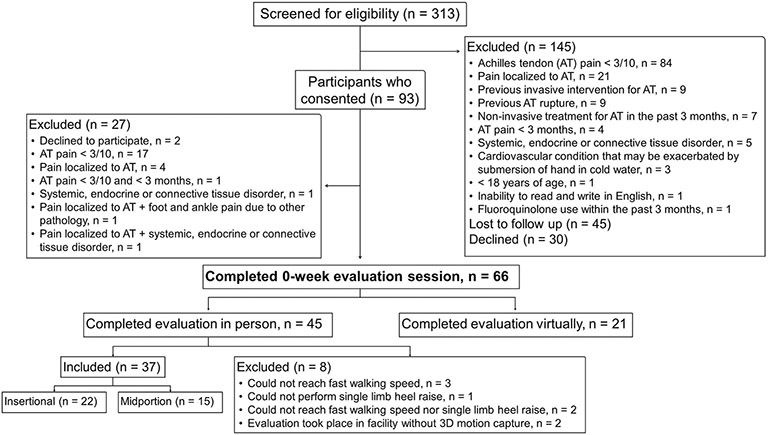
From September 2019 to December 2020, individuals with chronic AT were recruited via emails to the university community, databases of participants who had previously participated in research, referrals from the Department of Orthopaedics and Rehabilitation, and review of medical records. Baseline data were collected during a single two-hour visit to a hospital-based laboratory. Due to COVID-19, in-person human research was put on hold from March 17th, 2020, to July 16th, 2020. Also, from November 18th, 2020 to December 14th, 2020, in-person research was completed in a laboratory without a 3D motion capture system due to suspension of research within hospital-based settings. The inclusion criteria were a diagnosis of AT, defined as MeP ≥3/10 that was localized to the Achilles tendon insertion or midportion. A clinical exam supplemented by ultrasound imaging was used to rule out common differential diagnoses, including paratenonitis, tendon rupture, posterior ankle impingement, isolated bursitis, sural nerve injury, or lumbar radiculopathy. Only participants who completed 3D motion measurements at baseline (n = 37) were included in the current analysis. Reasons for exclusion from participation and the analysis are listed in Figure 1.
Figure 1:
Flowchart diagram indicating the number of individuals screened for eligibility, the number of participants consented, and the number of individuals included for analysis. The reasons for exclusion at each stage are provided, as detailed in a previous manuscript (Chimenti et al, 2023)
2.2. Demographics
Demographic measures, including sex, age, body mass index (BMI), and AT type (insertional or midportion) were collected at baseline. Participants were asked to quantify the duration of Achilles tendon morning stiffness (from 0 minutes to >100 minutes in 10-minute blocks) after getting up at the beginning of the day.
2.3. Pain
Participants rated their pain at rest and during several activities (MeP) using an 11-point verbal numeric rating scale (NRS) where 0 indicates “no pain” and 10 represents “the worst pain imaginable” (Kahl & Cleland, 2005). Participants rated their Achilles tendon pain on their most symptomatic leg. The task progression included: resting in a seated position with ankle in a neutral position; standing with equal weight on both feet; fast walking; single-leg heel raise endurance test; and standing calf stretch. Individuals were asked to rate the intensity of pain immediately after each task.
2.4. Sagittal-plane Biomechanics
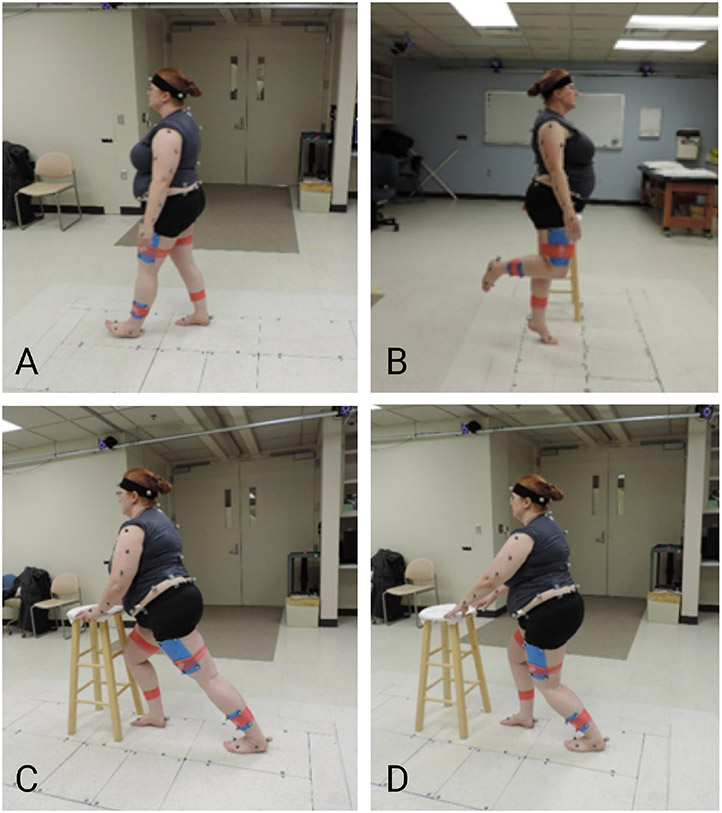
Kinematics were captured using a 12-camera system (Vicon Motion Systems of Centennial, CO and Los Angeles, CA) with 57 reflective markers, as described (Post et al., 2020; Wilken et al., 2012), and ground reaction forces were collected using 3 force plates (Advanced Mechanical Technology, Inc, Watertown, MA). For standing, individuals stood with equal weight distribution for 5 minutes during calibration. Then, they were asked to walk at a fast, standardized pace (Froude 4) (Esposito et al., 2014) across a level surface. Peak ankle dorsiflexion, peak knee extension, and peak hip extension were identified during the end of the stance phase. A minimum of three gait cycles were used to create a representative trial. Peak ankle power was calculated using inverse dynamics as the product of the net ankle moment and ankle angular velocity. Finally, peak ankle dorsiflexion angle was assessed during a standing lunge with the knee bent and fully extended. Each position was held for 3-5 seconds (Figure 2).
Figure 2:
Activities performed during biomechanical analysis. A) Walking; B) Single leg heel raise; C) Calf stretch: knee straight; D) Calf stretch: knee bent
2.5. Pain-related psychological questionnaires
Pain-related psychological questionnaires were completed immediately following the tendon loading and stretching tasks. Participants were instructed to think about any pain or discomfort that they had in the Achilles tendon during walking and heel raises while completing the Tampa Scale of Kinesiophobia (TSK-17) (Vlaeyen et al., 1995) and the Pain Catastrophizing Scale (PCS-13) (Sullivan et al., 1995).
2.6. Data Analyses
Two separate hierarchical linear mixed effects models were constructed. The first modeled pain intensity as a function of three levels of tendon loading tasks, i.e., resting, fast walking, and heel raises. The second modeled pain intensity as a function of tendon stretching tasks, i.e., resting, standing, and calf stretch. The two models were constructed and tested using the same methodology, with the only difference being the tasks included as independent variables. Models were developed to characterize MeP during varying intensities of tendon loading and stretching tasks, relative to pain at rest. Demographic, biomechanical, and psychological variables were then tested for association with pain intensity in the two models. As a quantitative variable for tendon loading, resting was assigned a value of 0, fast walking was assigned a value of 1, and heel raises a value of 2. For tendon stretching, resting was assigned a value of 0, standing a value of 1, and calf stretch a value of 2. It was tested with both linear and quadratic fits to ensure an appropriate assignment of discrete values. Our assessment confirmed the best fit was assigning task as a quantitative variable determined by partial F-tests. To test whether random effects significantly improved model fit, we considered both random intercept and combined random intercept and slope.
Modified univariate analyses, stepwise selection, and model validation was performed for both models. To determine which covariates to add to the initial models, we performed univariate tests for associations between MeP and demographic, biomechanical, and psychological variables.
Demographic, biomechanical, psychological, and functional variables that demonstrated a univariate p-value of < 0.3 were advanced to the next stage of model building and then included in stepwise selection for each model. Variables were tested for final model inclusion in ascending order starting with the lowest p-value from the univariate analyses. Best fit models were determined by the inclusion of variables achieving a p-value of < 0.05 when included during stepwise selection as well as the overall model demonstrating the lowest Akaike Information Criterion (AIC).
Both models were validated using Q-Q plots, residual plots, and histograms of the Pearson residuals to test for the normality of residuals and model fit. The plots demonstrated normal residual distribution and valid model fit for both models. Statistical analyses were performed using R 3.6.3 (R Core Team 2020) and the lme4 package (Bates et al., 2015).
3. Results
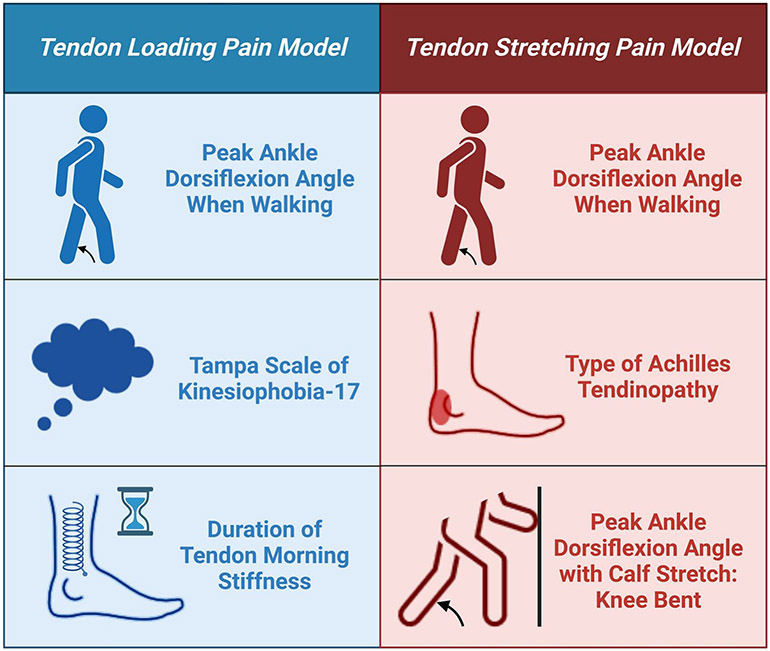
Demographic and clinical characteristics are shown in Table 1. Key variables associated with pain intensity in each model are summarized in Figure 3.
Table 1.
Characteristics of participants stratified by Achilles Tendinopathy (AT) type.
| Variables | All participants (n = 37) |
Insertional AT (n = 22) |
Midportion AT (n = 15) |
95 % CI |
|---|---|---|---|---|
| Demographics | ||||
| Sex female, n (%) | 20 (54) | 12 (54.5) | 8 (53.3) | - |
| Body Mass Index (kg/m2), mean ± SD | 28.9 ± 6.7 | 30.5 ± 7.2 | 26.4 ± 5.3 | −8.2, 0.1 |
| Age (years), mean ± SD | 44.1 ± 15.1 | 46.5 ± 15.7 | 40.5 ± 13.9 | −15.9, 4.0 |
| Tendon morning stiffness (1 unit = 10 minutes), mean ± SD | 3.7 ± 2.9 | 3.6 ± 2.8 | 3.7 ± 3.2 | −2, 2.2 |
| Pain (Numeric Rating Scale, 0-10), mean ± SD | ||||
| Pain at rest | 1.1 ± 1.4 | 1.4 ± 1.7 | 0.7 ± 0.7 | −1.6, 0.1 |
| Pain with fast walking | 3.3 ± 1.9 | 3.3 ± 2.0 | 3.2 ±1.8 | −1.4, 1.2 |
| Pain with heel raises | 4.5 ± 1.8 | 4.7 ± 1.8 | 4.3 ±1.9 | −1.7, 0.9 |
| Pain with standing | 1.9 ± 1.7 | 2.3 ± 1.9 | 1.3 ±1.19 | −2, 0 |
| Pain with calf stretch | 3.9 ± 2.5 | 4.2 ± 2.5 | 3.4 ± 2.5 | −2.4, 0.9 |
| Biomechanics | ||||
| Peak ankle dorsiflexion angle when walking (°) | 11.4 ± 2.8 | 11.6 ± 2.8 | 11.0 ± 2.8 | −2.5, 1.3 |
| Peak knee extension angle when walking (°) | −1.0 ± 6.4 | −0.3 ± 7.9 | −2.2 ± 3.3 | −5.8, 1.9 |
| Peak hip extension angle when walking (°) | −12.1 ± 8.7 | −11.5 ± 10.1 | −12.9 ± 6.2 | −6.8, 4.1 |
| Peak ankle power (J/s) | 3.1 ± 0.9 | 3.0 ± 0.9 | 3.2 ± 0.9 | −0.4, 0.9 |
| Peak ankle dorsiflexion angle with calf stretch: knee bent (°) | 32.3 ± 5.1 | 32.6 ± 5.5 | 32.0 ± 4.6 | −4, 2.8 |
| Peak ankle dorsiflexion angle with calf stretch: knee straight (°) | 28.7 ± 5.0 | 28.7 ± 4.9 | 28.8 ± 5.3 | −3.4, 3.7 |
| Psychosocial measures, mean ± SD | ||||
| Tampa Scale of Kinesiophobia-17 | 36.5 ± 5.2 | 37.1 ± 4.9 | 35.7 ± 5.7 | −5.1, 2.3 |
| Pain Catastrophizing Scale-13 | 9.6 ± 6.4 | 10.1 ± 6.3 | 8.8 ± 6.7 | −5.8, 3.2 |
Figure 3:
Variables significantly associated with pain intensity in the best fit tendon loading and tendon stretching pain models.
3.1. Tendon loading pain model
The best fit tendon loading pain model included the peak ankle dorsiflexion during walking, TSK-17 score, and duration of tendon morning stiffness, in addition to the term for task (rest, walking, heel raises) (Table 2). The fixed effects of the model accounted for 47% of the variance in pain intensity reported throughout the three tasks. Using a partial F-test, the inclusion of random slopes was not significant, and therefore, only random intercepts were utilized in the model. There was no group effect or interaction between insertional and midportion AT (Group: β=−0.76, P=0.34; Interaction: β=0.17, P=0.61) (Figure 4, Supplementary File A) and no association between peak ankle power and tendon loading tasks (β=−0.158, P=0.52). The model predicted a 1.7-point increase in pain intensity with walking from resting pain level and a 3.4-point increase in pain intensity with heel raises from resting pain level (Figure 5). Peak ankle dorsiflexion during walking had the strongest association with changes in pain intensity across tasks, suggesting that a 10° lower peak ankle dorsiflexion angle was associated with a 1.9-point greater pain intensity across loading tasks. Similarly, a 12-point higher score in the TSK-17 or a duration of 50 minutes of tendon morning stiffness were associated with a 1-point increase in pain intensity. Detailed results for this model and for the modified univariate analysis are listed in Table 2 and Supplementary File B.
Table 2.
Detailed output for the best-fit tendon loading model, including beta coefficients and 95% confidence intervals, t-values, and P values for each variable.
| Variable | β | β Cl | t-value | P value |
|---|---|---|---|---|
| Intercept | −0.267 | −4.84, 0.881 | −0.191 | < 0.001 |
| Peak ankle dorsiflexion angle when walking | −0.187 | −0.305, −0.069 | −3.18 | 0.003 |
| Tampa Scale of Kinesiophobia-17 | 0.082 | 0.018, 0.145 | 2.60 | 0.013 |
| Duration of tendon morning stiffness | 0.183 | 0.07, 0.296 | 3.26 | 0.002 |
| Loading tasks | 1.714 | 1.39, 2.04 | 10.58 | < 0.001 |
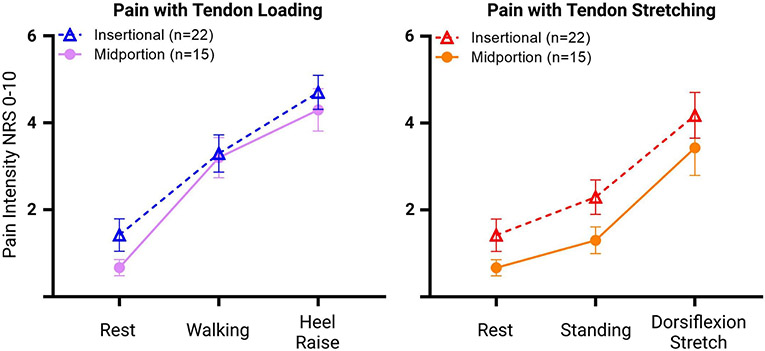
Figure 4:
Means (with standard errors) of reported pain by individuals with insertional vs midportion AT during rest, walking, and heel raises (tendon loading pain model, left), and during rest, standing, and calf stretch (tendon stretching pain model, right).
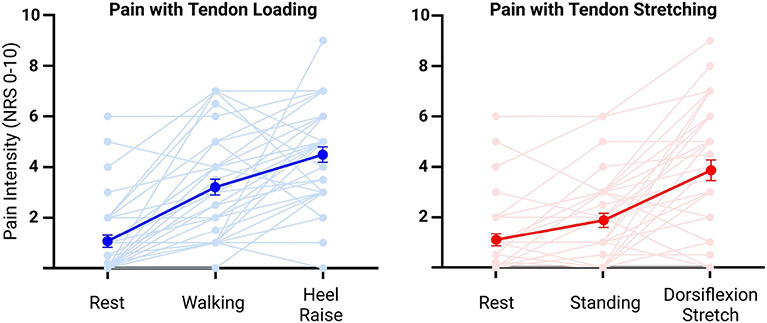
Figure 5:
Spaghetti plot of pain intensities for all 37 participants (light colors) along with the means and standard errors (dark colors) for the tendon loading pain model (left) and the tendon stretching pain model (right).
3.2. Tendon stretching pain model
The best fit tendon stretching model included AT type, peak ankle dorsiflexion angle during walking, and peak calf stretch angle, in addition to the term for task (rest, standing, calf stretch) (Table 3). The inclusion of the quadratic term with task as a quantitative variable provided the best fit determined by partial F-tests. The fixed effects of the model accounted for 53% of the variance in pain intensity throughout the three tasks. The best fit for random effects included terms for both random intercepts and random slopes. There was a significant group effect for the type of AT (β=−0.93, P=0.01), but no interaction between AT type and tendon stretching tasks (X2=0.28, P=0.86) (Figure 4, Supplementary File C). The model predicted a 0.8-point increase in pain intensity during standing from resting pain level as well as a 2.8-point increase in pain intensity with calf stretch from resting pain level (Figure 5). Peak ankle dorsiflexion angle during walking demonstrated the strongest association with pain intensity across tasks, suggesting that 10 degrees less peak ankle dorsiflexion angle was associated with 2.7-points greater tendon stretching pain. Also, a 10° greater ankle dorsiflexion stretch ROM was associated with a 1-point higher pain intensity across tasks. Insertional AT was associated with a nearly 1-point higher pain throughout tasks relative to midportion AT. Detailed results for this model and for the modified univariate analysis are listed in Table 3 and Supplementary File D.
Table 3.
Detailed output for the best-fit tendon loading model, including beta coefficients and 95% confidence intervals, t-values, and p-values for each variable.
| Variable | β | β Cl | t- value |
P value |
|---|---|---|---|---|
| Intercept | 5.27 | 1.0, 9.53 | 2.49 | 0.017 |
| Type of Achilles Tendinopathy | −0.93 | −1.65, −0.21 | −2.6 | 0.013 |
| Peak ankle dorsiflexion angle when walking | −0.27 | −0.41, −0.14 | −4.23 | <0.001 |
| Peak ankle dorsiflexion angle with calf stretch: knee bent | 0.095 | 0.02, 0.16 | 2.67 | 0.011 |
| Stretching tasks | 0.174 | −0.79, 1.15 | 0.36 | 0.72 |
| (Stretching tasks)2 | 0.604 | 0.146, 1.063 | 2.65 | 0.012 |
4. Discussion
Our findings demonstrate that MeP has a multifactorial presentation in individuals with chronic AT, where type of AT, biomechanical, and psychological measures were associated with the intensity of MeP. As hypothesized, MeP increased by a clinically meaningful amount with more demanding tasks (Salaffi et al., 2004), including activities commonly included in home exercise programs for AT. Also as hypothesized, AT type was associated with MeP intensity, as participants with insertional AT reported more severe MeP during tendon stretching tasks compared to those with midportion AT. Yet there were similar levels of MeP during tendon loading. Our hypotheses on the contribution of lower extremity biomechanics were partially supported with peak ankle dorsiflexion angle, but not ankle power. Our hypotheses on the contribution of fear of movement were also partially supported with fear of movement associated with MeP during tendon loading tasks and not during stretching tasks.
4.1. Sagittal-plane Biomechanics
The current study builds a more nuanced discussion on the influence of sagittal-plane ankle biomechanics on MeP in individuals with AT. Individuals with greater peak ankle dorsiflexion during walking reported lower MeP throughout both tendon loading and stretching tasks. In contrast, individuals with greater peak ankle dorsiflexion during the calf stretch reported higher MeP during tendon stretching tasks. We speculate that lower MeP during functional tasks contributes to greater use of mid-range ankle dorsiflexion (5° to 15°), while stretching into end-range dorsiflexion (>20°) aggravates MeP in this population. This difference between the impact of mid-range vs. end-range dorsiflexion on MeP aligns with previous research, where greater end-range dorsiflexion during stair ascent was associated with higher pain intensity during this task (Chimenti et al., 2016).
Overall participants reported an increase in MeP as the task intensity increased. In contrast, Sancho et al. (2019) reported no association between Achilles tendon forces and pain across 12 rehabilitation exercises in male runners with AT. The seemingly different findings may be due to the current study: 1) examining the change rather than a single pain rating, 2) selecting a few tasks that drastically increased in intensity from complete rest to a single leg calf endurance test rather than a continuum of 12 tendon-loading tasks, and 3) a patient population representing a more general population rather than focused on male runners. Consistent with the study by Sancho et al., we found no association between the magnitude of force, i.e. peak ankle power, and pain. Together, both studies support the idea that the level of force alone does not determine the intensity of MeP, which has multiple contributing factors.
4.2. Insertional vs. Midportion AT
Little is known about differences in MeP between those with insertional or midportion AT beyond the location of pain within the tendon. We found greater MeP throughout stretching tasks in participants with insertional AT (main effect of 0.9-points). Of note, no interaction was present between AT type and task in the tendon stretching model which suggests that although individuals with insertional AT had more severe pain than individuals with midportion AT, both groups demonstrated equal increases in pain with calf stretching. This finding may partially explain the efficacy of heel lifts to relieve midportion AT pain (Rabusin et al., 2021). There was also variability in MeP across AT types, indicating that some individuals found stretching to relieve pain while others found stretching to increase pain. These findings support the clinical rationale of initially avoiding the combination of tendon loading and stretching tasks as one strategy to minimize MeP during a progressive tendon loading exercise program. Moreover, this strategy may be helpful for not only individuals with insertional AT but also for some individuals with midportion AT as well.
4.3. Pain-related psychological factors
In general, higher MeP is associated with elevated fear of movement and pain catastrophizing in individuals with musculoskeletal pain (Leemans et al., 2022). In the current study we found the association between MeP and elevated fear of movement to be specific to dynamic activities with the tendon loading MeP model rather than static tasks. The average score of the TSK-17 was at the 37-point threshold for high fear of movement (Vlaeyen et al., 1995), while the average score for the PCS-13 was well below the 30-point threshold for elevated pain catastrophizing (Sullivan et al., 1995). This may explain the lack of association between pain catastrophizing and MeP in the current study. In addition, both insertional and midportion AT subgroups included a mix of individuals with elevated pain-related psychological factors and individuals without elevated pain-related psychological factors. Therefore, a clinical classification model that identifies a psychosocial-driven subtype, as described by Hanlon et al. (2021) may inform care beyond subtyping based on location of AT pain. Exercise and pain education have been shown to reduce pain-related psychological symptoms for adults with chronic musculoskeletal pain (Watson et al., 2019) and with AT pain (Chimenti et al., 2023). Further work is needed to understand how to best target interventions to address key factors contributing to an individual’s specific pain experience.
4.4. Strengths and Limitations
The current study is novel as it proposes several factors that may contribute to MeP, a primary symptom of AT and an emerging area of research within pain science (Fullwood et al., 2021). Also, this is the first study to examine the associations between biomechanical measures during walking and MeP in chronic AT. Yet, as a secondary analysis, no formal power calculations were performed to ensure adequate sample size for the proposed analysis. The sample size was limited by the number of participants who completed 3D motion analysis in person. We attempted to maximize the available sample size by accounting for task as a quantitative variable to minimize the number of terms in our models. Future studies with a larger sample size may be powered to detect other contributing factors to MeP.
A strength of the study is the generalizability of the findings to the general population as opposed to narrowly focusing on athletes. However, a resulting limitation is that pain intensity was measured during the relatively less demanding tendon loading tasks of heel raises and fast walking, as opposed to hopping and running. Nine of the included 37 participants could not perform single leg hopping at baseline; thus, it was not included in the statistical models due to the high rate of missing data. Additional research is needed to determine the effects of frontal and transverse plane motions during walking as well as ankle biomechanics during demanding tendon loading activities on MeP. This could provide evidence to guide decisions on using adjunct treatment, such as orthotics or bracing, to modulate tendon loading and pain in Achilles tendinopathy.
5. Conclusions
The present findings suggest that MeP is multifactorial in individuals with AT and seems to be associated with AT type, ankle biomechanics, and psychological measures. While individuals with insertional AT experienced more intense MeP with stretching compared to individuals with midportion AT, both insertional and midportion AT reported an increase in MeP intensity with tendon loading and stretching activities compared to rest. Greater use of mid-range ankle dorsiflexion during walking was associated with less severe MeP with both tendon loading and stretching tasks, indicating that limited functional use of ankle dorsiflexion angle is associated with more severe AT pain. For tendon loading activities, greater fear of movement is associated with greater MeP. Together these findings highlight factors that may aggravate MeP and should be considered when managing patients with Achilles tendinopathy.
Supplementary Material
Highlights.
Limited use of ankle dorsiflexion is associated with more severe pain in Achilles tendinopathy.
No association was found between movement-evoked pain and ankle power during fast walking.
Achilles tendinopathy type seems to influence movement-evoked pain and ankle biomechanics.
Acknowledgments
This work is supported by the National Institute of Arthritis Musculoskeletal and Skin Disease (NIAMS) research grant R00 AR071517 and by the Collaborative Research Grant from the International Association for the Study of Pain (IASP). Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1TR002537 and UL1TR002537. This research was funded in part by a Promotion of Doctoral Studies (PODS) The authors scholarship from the Foundation for Physical Therapy Research, and a Fulbright Scholarship (PRX21/00179), Ministry of Universities, Spain. These funding sources had no role in study design, collection, analysis/interpretation of data, or decision on submission for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ruth L. Chimenti reports financial support was provided by National Institute of Arthritis Musculoskeletal and Skin Disease (NIAMS). Ruth L. Chimenti reports financial support was provided by Collaborative Research Grant from the International Association for the Study of Pain (IASP). Alberto M. Heredia-Rizo reports financial support was provided by Fulbright Program, Ministry of Universities, Spain. Andrew A. Post reports financial support was provided by Promotion of Doctoral Studies (PODS). Ruth L. Chimenti reports financial support was provided by National Center for Advancing Translational Sciences of the National Institutes of Health. Kathleen A. Sluka reports a relationship with Pfizer Health AB that includes: consulting or advisory. Kathleen A. Sluka reports a relationship with Novartis Consumer Health that includes: consulting or advisory. Kathleen A. Sluka reports a relationship with GSK Consumer Healthcare srl that includes: consulting or advisory.
7. References
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Beyer R, Kongsgaard M, Hougs Kjaer B, Ohlenschlaeger T, Kjaer M, & Magnusson SP (2015). Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: A randomized controlled trial. Am J Sports Med. 43(7), 1704–1711. 10.1177/0363546515584760 [DOI] [PubMed] [Google Scholar]
- Chimenti RL, Flemister AS, Tome J, McMahon JM, & Houck JR (2016). Patients with insertional Achilles tendinopathy exhibit differences in ankle biomechanics as opposed to strength and range of motion. J Orthop Sports Phys Ther. 46(12), 1051–1060. 10.2519/jospt.2016.6462 [DOI] [PubMed] [Google Scholar]
- Chimenti RL, Bucklin M, Kelly M, Ketz J, Flemister AS, Richards MS, & Buckley MR (2017). Insertional achilles tendinopathy associated with altered transverse compressive and axial tensile strain during ankle dorsiflexion. Journal of orthopaedic research : official publication of the Orthopaedic Research Society, 35(4), 910–915. 10.1002/jor.23338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimenti RL, Post AA, Silbernagel KG, Hadlandsmyth K, Sluka KA, Moseley GL, & Rio E (2021). Kinesiophobia severity categories and clinically meaningful symptom change in persons with Achilles tendinopathy in a cross-sectional study: Implications for assessment and willingness to exercise. Front Pain Res (Lausanne), 2, 739051. 10.3389/fpain.2021.739051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimenti RL, Post AA, Rio EK, Moseley GL, Dao M, Mosby H, Hall M, de Cesar Netto C, Wilken JM, Danielson J, Bayman EO, & Sluka KA (2023). The effects of pain science education plus exercise on pain and function in chronic Achilles tendinopathy: A blinded, placebo-controlled, explanatory randomized trial. Pain, 164(1), 47–65. 10.1097/j.pain.0000000000002720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett DB, Simon CB, Manini TM, George SZ, Riley JL 3rd, & Fillingim RB (2019). Movement-evoked pain: transforming the way we understand and measure pain. Pain, 160(4), 757–761. 10.1097/j.pain.0000000000001431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos R-J, van der Vlist AC, Zwerver J, Meuffels DE, Smithuis F, van Ingen R, van der Giesen F, Visser E, Balemans A, & Pols M (2021). Dutch multidisciplinary guideline on Achilles tendinopathy. Br J Sports Med. 55(20), 1125–1134. 10.1136/bjsports-2020-103867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling GJ, Murley GS, Munteanu SE, Smith MM, Neal BS, Griffiths IB, Barton CJ, & Collins NJ (2014). Dynamic foot function as a risk factor for lower limb overuse injury: a systematic review. J Foot Ankle Res, 7(1),53. 10.1186/s13047-014-0053-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito ER, Rodriguez KM, Rabago CA, & Wilken JM (2014). Does unilateral transtibial amputation lead to greater metabolic demand during walking? J Rehabil Res Dev. 51(8), 1287–1296. 10.1682/JRRD.2014.06.0141 [DOI] [PubMed] [Google Scholar]
- Franceschi F, Papalia R, Paciotti M, Franceschetti E, Di Martino A, Maffulli N, & Denaro V (2014). Obesity as a risk factor for tendinopathy: a systematic review. Int J Endocrinol., 670262. 10.1155/2014/670262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullwood D, Means S, Merriwether EN, Chimenti RL, Ahluwalia S, & Booker SQ (2021). Toward understanding Movement-evoked Pain (MeP) and its measurement: A scoping review. Clin J Pain, 37(1), 61–78. 10.1097/AJP.0000000000000891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon SL, Pohlig RT, & Silbernagel KG (2021). Beyond the diagnosis: using patient characteristics and domains of tendon health to identify latent subgroups of Achilles tendinopathy. J Orthop Sports Phys Ther. 51(9), 440–448. 10.2519/jospt.2021.10271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasani F, Haines T, Munteanu SE, Schoch P, Vicenzino B, & Malliaras P (2021). LOAD-intensity and time-under-tension of exercises for men who have Achilles tendinopathy (the LOADIT trial): a randomised feasibility trial. BMC Sports Sci Med Rehabil, 13(1), 57. 10.1186/s13102-021-00279-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson P, Alfredson H, Sunding K, Fahlstrom M, & Cook J (2008). New regimen for eccentric calf-muscle training in patients with chronic insertional Achilles tendinopathy: results of a pilot study. Br J Sports Med. 42(9), 746–749. 10.1136/bjsm.2007.039545 [DOI] [PubMed] [Google Scholar]
- Kahl C, & Cleland JA (2013). Visual analogue scale, numeric pain rating scale and the McGill pain Questionnaire: an overview of psychometric properties. Phys Ther Reviews, 10(2), 123–128. 10.1179/108331905x55776 [DOI] [Google Scholar]
- Leemans L, Polli A, Nijs J, Wideman T, den Bandt H, & Beckwée D (2022). It Hurts to Move! Intervention Effects and Assessment Methods for Movement-Evoked Pain in Patients With Musculoskeletal Pain: A Systematic Review with Meta-analysis. The Journal of orthopaedic and sports physical therapy, 52(6), 345–374. 10.2519/jospt.2022.10527 [DOI] [PubMed] [Google Scholar]
- Magnan B, Bondi M, Pierantoni S, & Samaila E (2014). The pathogenesis of Achilles tendinopathy: a systematic review. Foot Ankle Surg. 20(3), 154–159. 10.1016/j.fas.2014.02.010 [DOI] [PubMed] [Google Scholar]
- Ogbonmwan I, Kumar BD, & Paton B (2018). New lower-limb gait biomechanical characteristics in individuals with Achilles tendinopathy: A systematic review update. Gait & posture, 62, 146–156. 10.1016/j.gaitpost.2018.03.010 [DOI] [PubMed] [Google Scholar]
- Post AA, Rio EK, Sluka KA, Moseley GL, Bayman EO, Hall MM, de Cesar Netto C, Wilken JM, Danielson JF, & Chimenti R (2020). Effect of pain education and exercise on pain and function in chronic Achilles tendinopathy: Protocol for a Double-blind, placebo-controlled randomized trial. JMIR Res Protoc, 9(11), e19111. 10.2196/19111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- Rabusin CL, Menz HB, McClelland JA, Evans AM, Malliaras P, Docking SI, Landorf KB, Gerrard JM, & Munteanu SE (2021). Efficacy of heel lifts versus calf muscle eccentric exercise for mid-portion Achilles tendinopathy (HEALTHY): a randomised trial. Br J Sports Med., 55(9), 486–492. 10.1136/bjsports-2019-101776 [DOI] [PubMed] [Google Scholar]
- Rompe JD, Furia J, & Maffulli N (2008). Eccentric loading compared with shock wave treatment for chronic insertional achilles tendinopathy. A randomized, controlled trial. J Bone Joint Surg Am. 90(1), 52–61. 10.2106/JBJS.F.01494 [DOI] [PubMed] [Google Scholar]
- Salaffi F, Stancati A, Silvestri CA, Ciapetti A, & Grassi W (2004). Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. European journal of pain (London, England), 8(4), 283–291. 10.1016/j.ejpain.2003.09.004 [DOI] [PubMed] [Google Scholar]
- Sancho I, Morrissey D, Willy RW, Barton C, & Malliaras P (2019). Education and exercise supplemented by a pain-guided hopping intervention for male recreational runners with midportion Achilles tendinopathy: A single cohort feasibility study. Phys Ther Sport 40, 107–116. 10.1016/j.ptsp.2019.08.007 [DOI] [PubMed] [Google Scholar]
- Silbernagel KG, Thomee R, Eriksson BI, & Karlsson J (2007). Continued sports activity, using a pain-monitoring model, during rehabilitation in patients with Achilles tendinopathy: a randomized controlled study. Am J Sports Med. 35(6), 897–906. 10.1177/0363546506298279 [DOI] [PubMed] [Google Scholar]
- Sluka KA, Frey-Law L, & Hoeger Bement M (2018). Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain, 159 Suppl 1(Suppl 1), S91–S97. 10.1097/j.pain.0000000000001235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, & Tan CW (2014). Effectiveness of the Alfredson protocol compared with a lower repetition-volume protocol for midportion Achilles tendinopathy: a randomized controlled trial. J Orthop Sports Phys Ther. 44(2), 59–67. 10.2519/jospt.2014.4720 [DOI] [PubMed] [Google Scholar]
- Sullivan MB,S; Pivik J (1995). The Pain Catastrophizing Scale: development and validation. Psychol Assessment, 7, 524–532. 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- Vallance P, Crowley L, Vicenzino B, & Malliaras P (2021). Contralateral mechanical hyperalgesia and altered pain modulation in men who have unilateral insertional Achilles tendinopathy: A cross-sectional study. Musculoskelet Sci Pract, 52, 102353. 10.1016/j.msksp.2021.102353 [DOI] [PubMed] [Google Scholar]
- van der Vlist AC, Breda SJ, Oei EHG, Verhaar JAN, & de Vos RJ (2019). Clinical risk factors for Achilles tendinopathy: a systematic review. Br Journal Sports Med. 53(21), 1352–1361. 10.1136/bjsports-2018-099991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaeyen J, K.-S. A, Boeren R, van Eek H (1995). Fearr of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain, 62, 363–372. [DOI] [PubMed] [Google Scholar]
- Watson JA, Ryan CG, Cooper L, Ellington D, Whittle R, Lavender M, Dixon J, Atkinson G, Cooper K, & Martin DJ (2019). Pain Neuroscience Education for Adults With Chronic Musculoskeletal Pain: A Mixed-Methods Systematic Review and Meta-Analysis. The journal of pain, 20(10), 1140.e1–1140.e22. 10.1016/j.jpain.2019.02.011 [DOI] [PubMed] [Google Scholar]
- Wilken JM, Rodriguez KM, Brawner M, & Darter BJ (2012). Reliability and Minimal Detectible Change values for gait kinematics and kinetics in healthy adults. Gait and Posture, 35(2), 301–307. 10.1016/j.gaitpost.2011.09.105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.