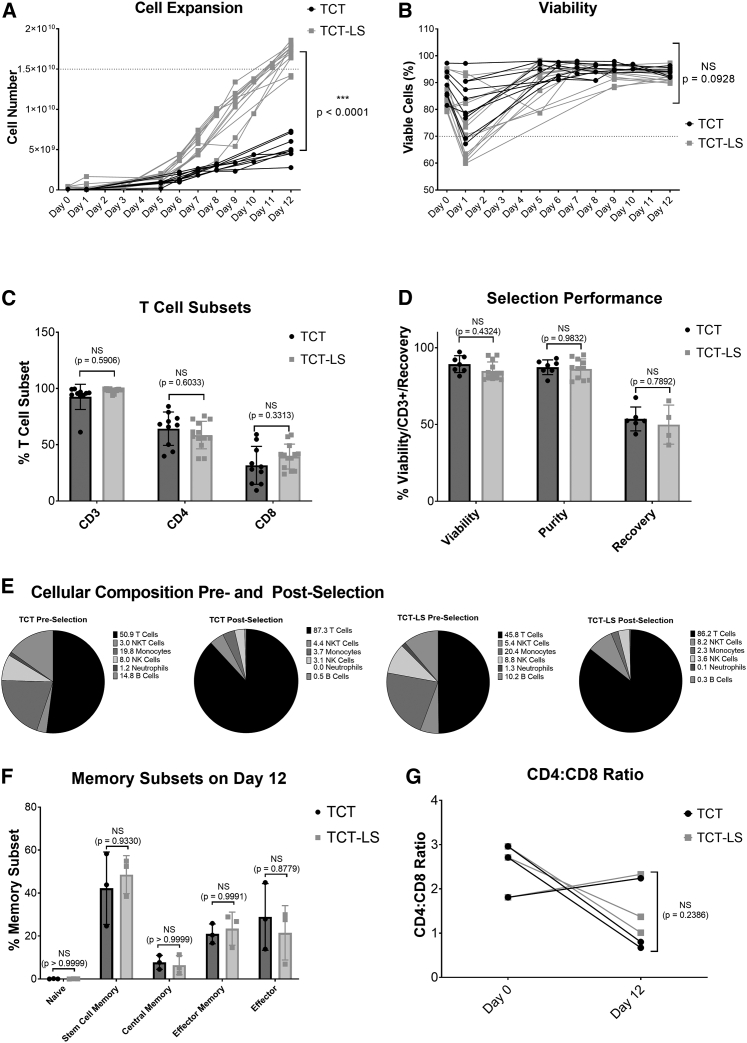
Figure 2.
Comparison of standard (TCT) and large-scale (TCT-LS) manufacturing processes
(A) Comparison of cell expansion over 12 days between TCT and TCT-LS processes (n = 10 for TCT and 13 for TCT-LS, individual data points shown, unpaired t test of day 12 values). (B) Comparison of viability over 12 days between TCT and TCT-LS processes (n = 10 for TCT and 13 for TCT-LS, individual data points shown, unpaired t test of day 12 values). (C) Comparison of %CD3+, %CD4+, or %CD8+ T cell subsets in drug product manufactured using either TCT or TCT-LS process (n = 10 for TCT and 13 for TCT-LS; graph shows individual data points with mean + SD; two-way ANOVA with Sidak’s multiple comparisons test). (D) Comparison of viability, purity (%CD3+ cells), and cell recovery during selection in TCT and TCT-LS processes (n = 7 for TCT and 13 for TCT-LS, graph shows individual data points with mean + SD; two-way ANOVA with Sidak’s multiple comparisons test). (E) Cellular composition pre and post selection for TCT and TCT-LS processes (n = 6 for TCT and 10 for TCT-LS, graph shows mean). (F) Comparison of memory T cell subsets in product manufactured using either TCT or TCT-LS in matched donors (n = 3, graph shows individual data points with mean + SD; two-way ANOVA with Sidak’s multiple comparisons test). (G) Comparison of CD4:CD8 ratio on day 0 and day 12 in product manufactured using either TCT or TCT-LS in matched donors (n = 3; graph shows individual data points with mean + SD; two-way ANOVA with Sidak’s multiple comparisons test).