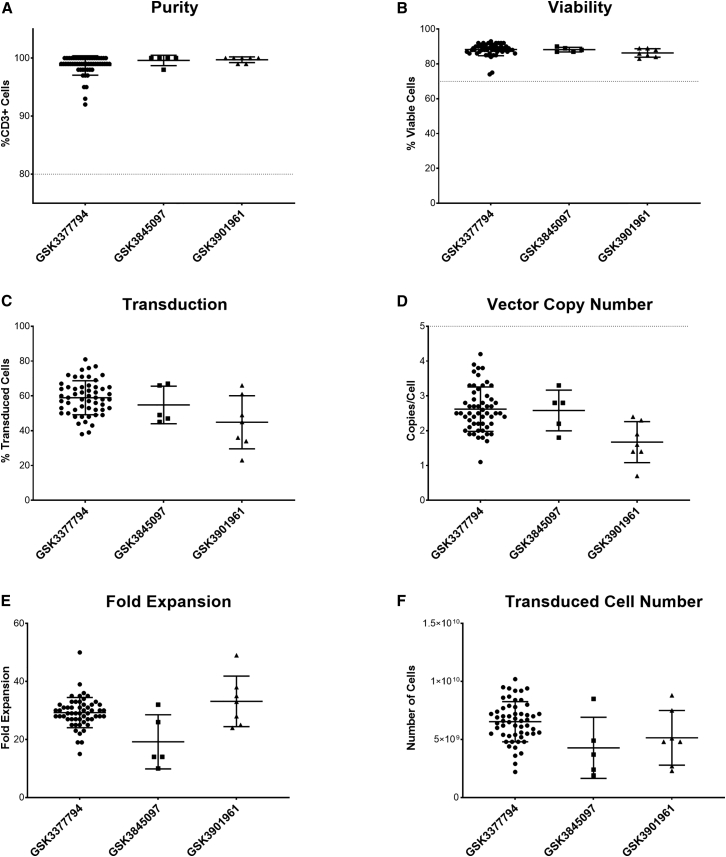
Figure 6.
Summary of clinical manufacturing data for three T cell products (n = 55 for GSK3377794, five for GSK3845097, and seven for GSK3901961)
(A) Purity (%CD3+ cells); acceptance criterion of 80% shown by dotted line. (B) Post-thaw viability; acceptance criterion of 70% shown by dotted line; GSK3377794 data are the same as post-thaw viability shown in Figure 3C. (C) Transduction efficiency. (D) Vector copy number; acceptance criterion of >5 copies/cell shown by dotted line. (E) Fold expansion (cells in final product/cells seeded for expansion). (F) Total number of transduced cells manufactured. Graphs show individual data points with mean + SD.