Abstract
A paralytic poliomyelitis outbreak occurred in Namibia in 2006, almost exclusively among adults. Nineteen cases were virologically confirmed as due to wild poliovirus type 1 (WPV1), and 26 were classified as polio compatible. Eleven deaths occurred among confirmed and compatible cases (24%). Of the confirmed cases, 97% were aged 15–45 years, 89% were male, and 71% lived in settlement areas in Windhoek. The virus was genetically related to a virus detected in 2005 in Angola, which had been imported earlier from India. The outbreak is likely due to immunity gaps among adults who were inadequately vaccinated during childhood. This outbreak underscores the ongoing risks posed by poliovirus importations, the importance of maintaining strong acute flaccid paralysis surveillance even in adults, and the need to maintain high population immunity to avoid polio outbreaks in the preeradication period and outbreaks due to vaccine-derived polioviruses in the posteradication era.
Keywords: Namibia, poliomyelitis, type 1 wild poliovirus, disease outbreak, paralysis
Since the launch of the Global Polio Eradication Initiative in 1988, significant progress has been made against polio, with a decrease of >99% in the number of polio cases and circulation of indigenous wild poliovirus (WPV) in only 3 remaining countries: Afghanistan, Nigeria, and Pakistan [1]. However, outbreaks due to WPV importations have occurred in previously polio-free countries in several regions [2–4]. Although only 2 previously polio-free countries experienced importations and outbreaks in 2012 (Niger and Chad) [1], concerns remain about countries that may have inadequate coverage with oral poliovirus vaccine (OPV). Gaps in population immunity put these countries at risk of WPV infections. We describe an outbreak of WPV type 1 (WPV1) infection in Namibia during 2006 that predominantly affected young adult males.
BACKGROUND
Namibia (2006 population, approximately 2.05 million) has the second lowest population density (1.7 people/km2) among African countries. With a 2011 per capita income of US$6960, Namibia is one of the wealthier African countries [5]. However, there is a high degree of income inequality in Namibia, as evidenced by its 2004 Gini coefficient of 0.64 (the Gini coefficient measures the extent to which the distribution of income among individuals or households deviates from a perfectly equal distribution, where a coefficient of 0 means perfect equality, and a coefficient of 1 implies perfect inequality; countries with a coefficient of ≥0.5 are considered to have high levels of income inequality) [6]. During the 20-year civil war preceding Namibia’s independence, in 1990, children had limited access to primary health care and immunization services. The Namibia Expanded Program on Immunization (EPI) was established in June 1990. During 1990–1995, EPI outreach activities targeting children aged <5 years were conducted quarterly. Routine infant immunization services were also strengthened, resulting in improvements in immunization coverage. According to the estimates from the World Health Organization and the United Nations Children’s Fund (UNICEF), in the first decade after EPI was established, routine infant coverage with at least 3 doses of oral poliovirus vaccine in Namibia ranged from 69% to 80% and increased to 82%, 81%, and 86% in 2003, 2004, and 2005 respectively [7], although 3-dose OPV coverage exceeded 80% in only 10 (30%) of 33 districts in 2005. Infant 3-dose OPV coverage reported in demographic health surveys was lower but increased from 64.6% in 1992 to 76.1% in 2000 and 76.5% in 2006–2007 [8–10]. Despite accelerated EPI activities, 2 outbreaks predominantly among children <5 years of age and caused by viruses linked to Angola were reported in Namibia in 1993–1995 [11, 12]. Since 1996, annual national immunization day campaigns targeting children aged <5 years have been held, with reported coverage of ≥90% in most of the 34 districts in the country, although independent monitoring was not done to validate administrative coverage data. Since 1995 until the outbreak in 2006, no WPV cases were reported.
Acute Flaccid Paralysis (AFP) Surveillance
Core performance indicators for AFP surveillance have been established to help ensure that surveillance systems are sensitive enough to detect poliomyelitis cases. In the 3 years (2003–2005) before the outbreak, AFP surveillance core performance indicators in Namibia reached certification standard levels as per global eradication requirements. Countries in WHO regions not certified as free of polio should achieve an annual rate of non–polio-associated AFP of ≥2 cases per 100 000 population aged <15 years. To ensure sufficiently complete and reliable laboratory analysis, ≥80% of AFP cases should have 2 stool specimens collected ≥24 hours apart, within 14 days of paralysis onset, arriving in good condition at an accredited Global Polio Laboratory Network laboratory.
Initial Laboratory-Confirmed Case
The onset of paralysis in the first case patient with laboratory-confirmed WPV infection was 6 May 2006. This occurred in a 39-year-old male farmer from Aranos district who underwent abdominal surgery a month prior in Windhoek. He developed sudden paralysis of both lower limbs while recuperating, followed by paralysis of respiratory muscles. He died from respiratory failure a month later. The attending physician suspected polio. Two stool specimens from the patient were tested in the World Health Organization-accredited laboratory at the National Institute for Communicable Disease (NICD; Johannesburg, South Africa) yielded WPV1.
METHODS
Initial Outbreak Investigation
An outbreak investigation team led by staff affiliated with the Namibia Ministry of Health and Social Services (MHSS) was conducted with additional technical support provided by the WHO and the Centers for Disease Control and Prevention (CDC) epidemiologists. Information on probable risk factors (eg, history of recent travel to or contact with persons from polio-infected countries and immunization status) was collected from relatives of the first reported case patient in the outbreak. Following laboratory confirmation of the first case, a search for missed cases of AFP was conducted in neighboring farms in Aranos province, and general AFP surveillance was intensified. Windhoek hospitals were visited for interviews with clinicians and other health workers to identify possible AFP cases. Outpatient and inpatient records were searched for possible missed cases and to validate reported cases. Surveillance trainings were held for region and district health staff, who were instructed to intensify AFP surveillance, perform retrospective case searches in health facilities, and report weekly to the central level. A line list of reported AFP cases and an epidemic curve were updated regularly at the central level. Core AFP surveillance performance indicators were analyzed weekly, with feedback provided to regions.
Case Definition
AFP cases were classified as laboratory-confirmed polio if WPV was isolated from a clinical specimen. AFP cases with 2 stool specimens collected within 14 days of onset of paralysis, that were negative for WPV, or that had specimens collected after 14 days and no residual paralysis at the 60-day follow-up examination were discarded as non–polio associated. Cases with inadequate stool specimens were reviewed by the National Polio Expert Committee (NPEC) and classified as “compatible” if there was residual paralysis at least 60 days following paralysis onset and clinical characteristics consistent with poliomyelitis. Cases with inadequate stool specimens and lost to 60-day follow-up were classified as non–polio associated by the NPEC if alternative diagnoses were made by the NPEC.
Outbreak Response
In response to the outbreak, Namibia’s Ministry of Health conducted 3 rounds of national immunization days, during 21–23 June, 18–20 July, and 22–24 August 2006. The first round performed 19 days after laboratory confirmation of the first recognized case, in accordance with the Advisory Committee on Polio Eradication recommendation for outbreak response within 4 weeks of detection [13]. Type 1 monovalent oral poliovirus vaccine was used during the first 2 rounds; trivalent OPV was used for the third round. Because of the age distribution of the cases, the first 2 national immunization days targeted adults and children of all ages; the third round targeted only children aged <5 years.
Administrative coverage for each of the 34 districts was calculated as the proportion of the total district population vaccinated during the first 2 rounds and the proportion of the population aged <5 years that was vaccinated for the third round. Target population figures were based on the projected population figures for 2006, provided by the MHSS.
One day after each of the 3 rounds, independent monitors visited randomly selected houses to assess the vaccination status of household members. Where at least 90% of the total assessed population in the households was vaccinated, as evidenced by the presence of indelible ink on fingers of vaccine recipients, coverage was considered adequate. Independent monitoring was confined to the Khomas and the northern regions, epicenters of the outbreak.
Case-Control Study
A case investigation form with questions about potential risk factors and clinical information was developed and used by MHSS staff and consultants to collect information about AFP cases from patients, families, and medical records. Risk factors were evaluated by comparing information from cases with laboratory-confirmed WPV infection to information from controls with non–polio-associated AFP. Compatible cases were not included in the analysis. Data were entered into an EpiInfo, version 3.3, database (CDC, Atlanta, GA), and analyzed using SAS, version 9.1 (SAS Institute, Cary, NC). For analysis of categorical variables, χ2 P values and 95% confidence intervals were calculated.
Settlement Survey
Because most WPV cases occurred among male residents of Katutura settlement areas, a cluster survey was conducted in these areas in October 2006 to better evaluate resident characteristics and risk behaviors, by sex. The main settlement areas in Katutura included 31 equally sized grids on the local administrative map. Grids were sequentially numbered from southeast to northwest. Fifteen sampling units were randomly selected and assumed to contain equal numbers of households. Recruitment targets were 7 households per sampling unit. Two local interviewers fluent in at least 1 of the dominant local languages conducted interviews. Households were selected using sequential visits to neighboring households near a randomly identified starting point. To minimize loss of out-of-area residents returning to outlying regions during weekends and to maximize opportunities to reach persons after working hours, survey work was completed during weekday hours of 4:30 PM and 7:30 PM. Data were analyzed in SUDAAN, version 10 (Research Triangle Institute, Research Triangle Park, NC).
Laboratory Methods
Stool specimens were processed in the WHO-accredited regional reference laboratory in Johannesburg, South Africa. Stool suspensions were prepared and inoculated onto 2 cell culture lines, using methods described in the WHO laboratory manual for poliovirus isolation [14]. The viruses were identified as type 1 poliovirus by use of virus neutralization with standardized reference antisera and were characterized as WPV by using standardized reverse-transcription polymerase chain reaction intratypic differentiation method. Type 1 WPVs identified were further characterized by partial genomic sequencing of the VP1 coding region, using previously described primers and cycle sequencing [15, 16]. Comparisons of VP1 sequences were made to other viruses from southwest Africa, using the neighbor-joining method as implemented in the program MEGA4 [17], and the dendrogram was rooted to an isolate from Angola that represented the shortest genetic distance from the Namibia viruses.
RESULTS
Outbreak Description
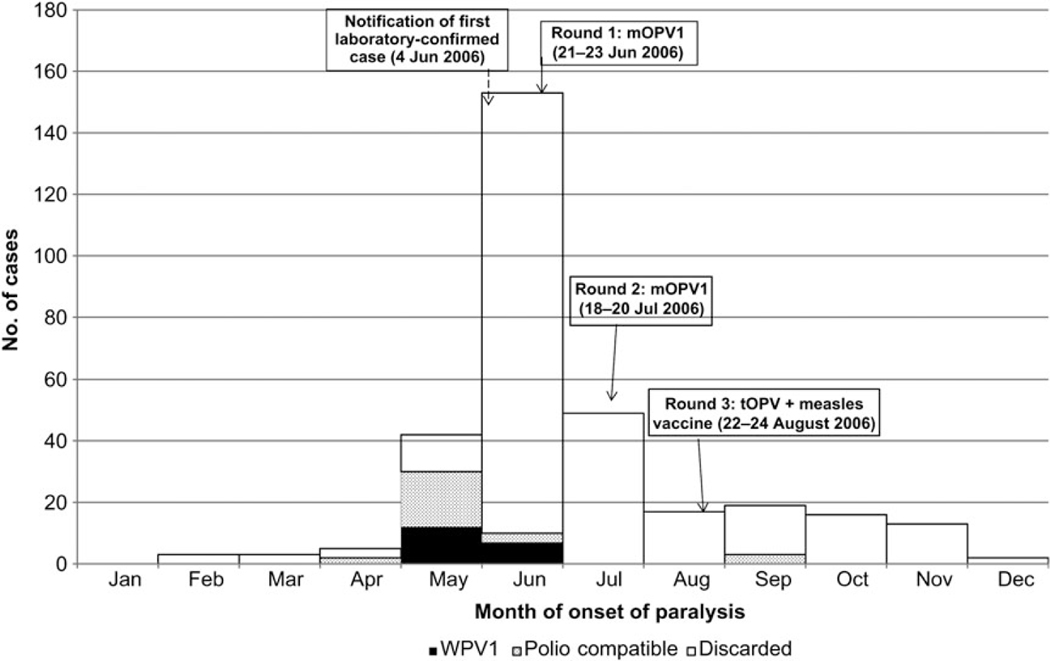
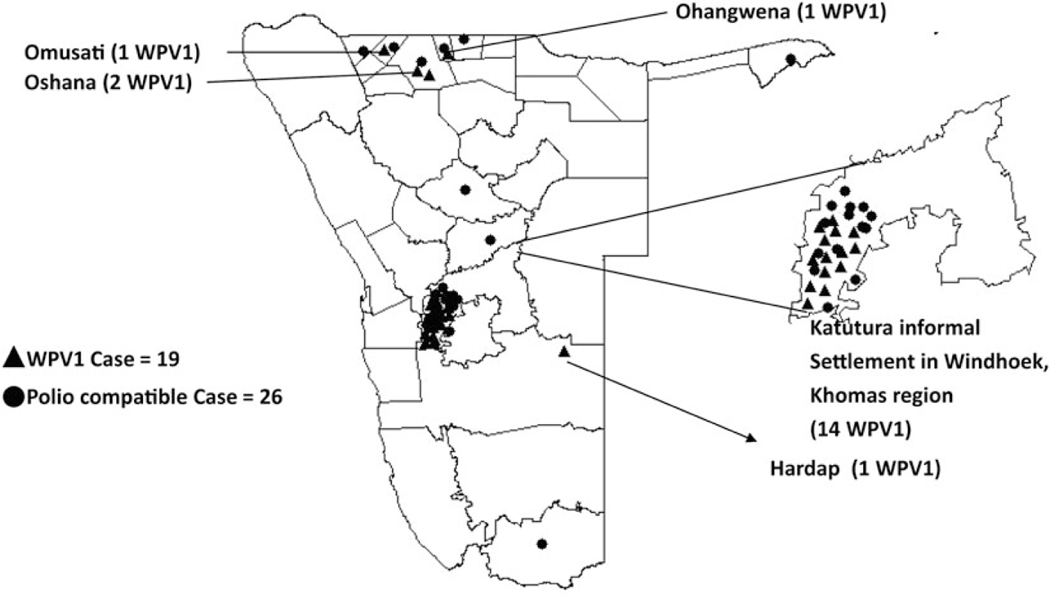
The outbreak included 277 non–polio-associated AFP cases, 19 laboratory-confirmed paralytic cases, and 26 polio-compatible cases (Figure 1). Onset of the last laboratory-confirmed case was 26 June 2006, and onset of the last compatible case was 13 September 2006. Two AFP cases with paralysis onset earlier than the first laboratory-confirmed case were classified as compatible. These 2 cases were from northern regions of Ohangwena and Omusati, indicating that the virus may have spread from the north along the common travel route to Windhoek, with further spread enhanced by travel between northern regions and Windhoek. Fourteen of 19 persons (77%) with WPV infection were residents of crowded Katutura squatter settlement areas on the outskirts of Windhoek. Five cases occurred in residents of 3 northern districts that share borders with Angola (Figure 2). The age of the 19 laboratory-confirmed paralytic cases was 14–59 years; 17 cases (90%) occurred in persons 15–29 years old. None occurred in children aged <14 years. Only 2 cases were female. Eleven deaths attributed to polio occurred among the confirmed and compatible cases (case-fatality ratio, 24%), of which 6 resulted from respiratory complications.
Figure 1.
Laboratory-confirmed wild poliovirus type 1 (WPV1) cases, polio-compatible cases, and “discarded” cases (non-polio acute flaccid paralysis [AFP] cases), by month of onset, Namibia, 2006.
Figure 2.
Laboratory-confirmed wild poliovirus type 1 (WPV1) cases and polio-compatible cases, Namibia, 2006.
Case-Control Analysis
Compared with controls, cases were significantly more likely (P < .05) to be male, to have been born in northern Namibia, to have had contact with Angolan nationals in the previous 3 months, to live in urban areas, and to have visited shebeens (informal alcohol-serving establishments; Table 1). Having received an intramuscular injection, having recent visitors to the household, toilet type, water source, household size, presence of children in the household, and contact with a paralyzed or ill person before onset were not significantly different between cases and controls.
Table 1.
Univariate Analysis of Risk Factors Among Individuals With Wild Poliovirus–Confirmed Paralysis (Cases) and Non–Polio-Associated Acute Flaccid Paralysis (Controls), Namibia, 2006
| Factor | Cases (n = 18) | Controls (n = 131) | OR (95% CI) | P Value |
|---|---|---|---|---|
|
| ||||
| Male sex | 16/18 | 55/117 | 9.0 (2.0–41.0) | .001 |
| Born in northern Namibia | 12/12 | 72/111 | ∞ (1.2–∞) | .01 |
| Contact with Angolan national past 3 mo | 6/10 | 20/108 | 6.6 (1.7–25.6) | .003 |
| Resides in urban area | 8/10 | 37/87 | 5.4 (1.1–27.0) | .02 |
| Visited shebeens | 11/12 | 41/106 | 17.4 (2.2–140.2) | .0005 |
| Intramuscular injection before onset paralysis | 2/9 | 10/115 | 3.0 (.6–16.4) | .18 |
| Visitors to household before onset | 5/12 | 28/120 | 2.4 (.7–8.0) | .16 |
| Use of bush/field for toileting | 7/13 | 64/121 | 1.0 (.3–3.3) | .38 |
| Use of piped water source | 10/14 | 86/127 | 1.2 (.4–4.0) | .80 |
| Household size of ≤5 persons | 9/15 | 46/122 | 2.5 (.8–7.4) | .10 |
| No children in household | 7/15 | 44/123 | 1.6 (.5–4.6) | .41 |
| Contact with paralyzed/sick person | 2/8 | 14/114 | 2.4 (.44–113.0) | .30 |
Data are individuals with the characteristic/individuals evaluated. For some factors, denominators differ from 18 and 131, owing to unknown/missing information.
Settlement Area Survey
Respondents from 102 households were interviewed. The mean household size was 5.4 persons. Of 518 reported residents, 47% were male. Most residents (80%) were ≥14 years old; >75% of households had no children <5 years of age. The majority of respondents (73%) reported being born in northern regions (Omusati, Oshana, Ohangwena, Oshikoto, Kavango, or Kunene) or in the Windhoek area. Male respondents (52%) were more likely than females (34%) to be born in the northern regions of Namibia than in Windhoek (P = .03; data not shown). A shebeen was in or connected to 16% of households. Male respondents were more likely than females to report a shebeen in the household (P = .005; data not shown). About half of respondents reported visiting a shebeen in the previous 30 days. Men were more likely than women to have slept or eaten at a shebeen (P = .04 and P = .05, respectively). Recent travel to and from northern Namibia was common (26% in past 30 days). Males (56%) were more likely than females (28%) to report contact with persons from the north, either through recent travel to or visitors from these regions (P = .02).
Mass Campaign Coverage
Administrative coverage for the 3 rounds of response mass campaign was >95% in >80% of the 34 districts in the country. Independent monitoring conducted in Khomas and northern regions confirmed administrative coverage rates, although these coverage figures cannot be generalized to the whole country.
Genetic Analysis of Viruses
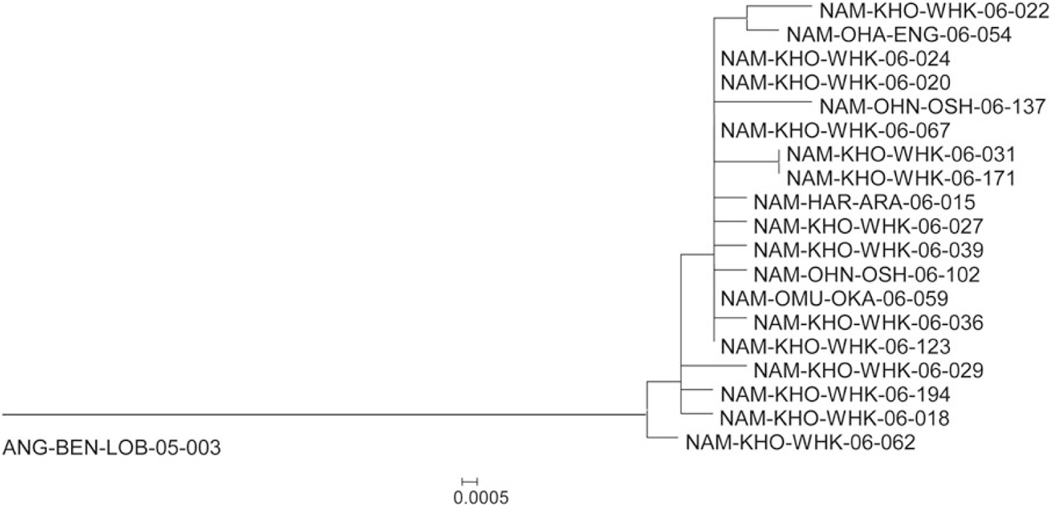
The dendrogram representing the VP1 genetic information of the viruses from this outbreak forms a single monophyletic branch connecting Namibian outbreak virus sequences to the sequence of the most closely related Angolan virus, which was first detected there in 2005 (Figure 3). The single branch is consistent with a single importation event into Namibia; however, the long length of the branch to the closest related viruses in Angola prevents precise timing of the importation event from the sequence information alone. The length of the branch and an assumption about the molecular clock [19] infers that the virus circulation before the detection of the first outbreak case in Namibia was missed by surveillance for approximately 2 years (ie, since 2004). It is likely that most of this missed circulation occurred in Angola; however, the split from the main branch by the single isolate from case NAM-KHO-WHK-06–062 is an indication that some circulation in early 2006 was possibly missed in Namibia, as well. The 4 isolates obtained from northern districts (1 each from Engela and Okahao and 2 from Oshakati; Supplementary Table 1) do not appear consistently ancestral on the dendrogram; therefore, they are more consistent with virus transmission between the 2 areas of the country. There is no clear indication of the location of the first virus infections, also consistent with spread from the area around Windhoek to the north during the course of the outbreak. The tree also indicates that the index case of the outbreak was not likely among the first infections following importation.
Figure 3.
VP1 phylogenetic relationships among wild poliovirus type 1 isolated from 19 cases in Namibia, based on analysis of the 906-nucleotide VP1 sequences. The tree was generated by the neighbor-joining algorithm, using the Kimura 2-parameter nucleotide substitution model [18] implemented in MEGA 4 [17].
DISCUSSION
This polio outbreak had 2 foci: Katutura settlement areas and northern Namibia regions. Our survey confirms that travel between Katutura and the north was common. Males were more likely than females to travel to or have visitors from the north, potentially increasing their risk for WPV infection. The sequence analysis also supports the frequent-travel hypothesis, since viruses from both areas were closely related along at least 2 separate chains of transmission. The precise location where virus was introduced into Namibia, however, is unknown because there is not a close genetic linkage to the Angola viruses, which may have circulated undetected following importation from northern India sometime in 2004 or 2005.
This outbreak demonstrates that populations of any age, including adults, with low immunity against poliovirus, are at risk. Lack of reliable information on vaccination history for most of the confirmed cases limits the ability to directly attribute the outbreak to immunization gaps. However, the outbreak primarily affected young adults born before 1990, persons who would have been too old to have been immunized in the outreach activities targeting children aged <5 years that were conducted 4 times per year during 1990–1995. Low 3-dose OPV coverage is confirmed by age-cohort analysis from a 1992 survey, in which 3-dose OPV coverage by 12 months of age was 37% among 4-year-old children, 38.1% among those aged 3 years, 55.1% among those aged 2 years, and 64.6% among those aged 1 year [8]. Improved 3-dose OPV coverage since 1990 likely explains the lack of cases among children. Additionally, the fact that almost 75% of the settlement households had no children aged <5 years suggests that, unlike most polio outbreaks, children were not major contributors to poliovirus transmission in Katutura.
Although 2 poliomyelitis outbreaks involving adults occurred in the 1990s, in Albania and the Netherlands [20, 21], most polio outbreaks primarily affect children. In the 1996 Albania outbreak, 70% of cases occurred in persons ≥15 years of age, probably due to a previous 2-dose infant vaccination schedule and cold chain problems. In the Netherlands outbreak (median age 24.5 years), cases occurred in persons who refused vaccination for religious reasons. This 2006 Namibia outbreak, a WPV1 outbreak in the Republic of Congo in 2010–2011 [4] in which 331 of 445 cases (73%) occurred in adults, a 2011 WPV outbreak in China in which 11 of 21 cases (52%) occurred in adults [3] , and a large WPV1 outbreak in Tajikistan in 2010 in which 159 of 461 (35%) of cases occurred in older children and adults [22] provide recent evidence that populations of any age with low immunity against poliovirus are at risk, demonstrating the need for vigilant AFP surveillance in all age groups. In Namibia, Tajikistan, and the Republic of Congo, civil conflict created low immunization coverage and resultant age-specific immunity gaps. Countries with current or past civil conflicts should assess the immunity status of age-specific cohorts and develop strategies to minimize the risk of outbreaks in children and adults.
The efficient WPV circulation in populations with suboptimal immunity, even in a country with low population density, demonstrates the importance of achieving and maintaining high immunity levels in children before eradication, to prevent future outbreaks in the posteradication world. The greatest risk for poliovirus circulation in the world after cessation of OPV use will be vaccine-derived poliovirus (VDPV) transmission within the first few years after OPV cessation [23]. Infections with VDPV had clinical paralytic attack rates similar to those for WPV infections in recent outbreaks in Indonesia and Nigeria [24, 25]. Evaluation of immunity status and vaccination of adult populations with low immunity is impractical on a global scale. Obtaining high immunity levels in children in the years before vaccination cessation will protect future adults, therefore providing the best protection against WPV and VDPV circulation.
Our data suggest that the preponderance of male WPV cases in the Katutura settlements in this outbreak was not due to sex-based distribution (ie, more case in men than women) in the area. However, more than half of the men, a higher proportion than women, were born in northern regions. Immunization coverage was lower in the north at the time that the age group affected by the outbreak would have been children [8, 9]. Being born in or having lived in the north as a child were also identified as risk factors in the case-control study.
Visiting shebeens was common among both men and women, but male respondents in the survey were more likely than women to have eaten or slept in shebeens. Recent visits to shebeens were also more common among cases than controls. Shebeens provide venues for socializing typically characterized by overcrowding and unhygienic conditions. Alcohol abuse, high-risk sexual behavior, human immunodeficiency virus transmission, and tuberculosis transmission have also been associated with shebeens [26–29]. The apparent efficiency of WPV circulation in a crowded settlement area with suboptimal sanitation and the opportunity for heightened person-to-person transmission in shebeens underscore the role of environmental and behavioral factors in polio transmission.
Low AFP surveillance sensitivity early in the outbreak and the retrospective identification of compatible cases with onset before onset of the first laboratory-confirmed case, suggest that the true index case may have been missed. Adequate stool collection between 1 January and 2 October 2006 was only 67% and exceeded 80% in only 4 of Namibia’s 13 regions. These suboptimal surveillance indicators suggest that the outbreak may have been more extensive. The high rate of non–polio-associated AFP (42.7 cases/100 000 persons) during the outbreak greatly exceeds the expected rate, raising the possibility that some cases of paralysis due to WPV infection may have been classified as non–polio-associated AFP cases. Three polio-compatible cases had onset of paralysis after the last laboratory-confirmed case in the outbreak, suggesting that WPV circulation may have continued beyond 26 June 2006, the onset of the last laboratory-confirmed case (Figure 1). This implies that delays in notification of cases and incomplete or delayed collection of specimens may have led to incomplete case identification, further underscoring the need to maintain vigilant AFP surveillance.
The long genetic distance of the Namibia outbreak virus from the closest Angola viruses indicates that WPV circulation in the southwest African subregion escaped detection for approximately 2 years, consistent with considerable AFP surveillance quality gaps in the northern Namibia–southern Angola epidemiological block. In the period before the outbreak, core AFP surveillance indicators were suboptimal in this area. Although it is not possible to say precisely where surveillance gaps occurred, historical risk assessment data would infer that they occurred at the subnational level in Angola and in northern Namibia. Maintaining sensitive surveillance is critical to enable prompt and accurate detection of poliovirus and rapid response.
The case-control analysis is subject to limitations. It is possible that some non–polio-associated AFP cases used as controls were unidentified WPV cases, a situation that would bias the results toward the null. However, we were still able to detect significant differences between cases and controls, enabling identification of some risk factors. Information on some risk factors was unavailable for several cases and controls, possibly leading to a bias if characteristics of cases or controls with missing information differed from those with information. Resource and time constraints limited the number of households that could be visited as part of the settlement survey. However, our purpose was more to elucidate potential risk factors rather than to provide generalizable census estimates.
This outbreak underscores the ongoing risks posed by poliovirus importations, the importance of maintaining strong AFP surveillance, including among adults, and the need to maintain high population immunity during the years before stopping vaccination, to protect against WPV and VDPV circulation both before and after eradication.
Footnotes
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Disclaimer. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of UNICEF, the WHO, the CDC, or the NICD.
Supplement sponsorship. This article is part of a supplement entitled “The Final Phase of Polio Eradication and Endgame Strategies for the Post-Eradication Era,” which was sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease C, Prevention. Progress toward eradication of polio–worldwide, January 2011-March 2013. MMWR Morb Mortal Wkly Rep 2013; 62:335–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease C, Prevention. Outbreaks following wild poliovirus importations — Europe, Africa, and Asia, January 2009-September 2010. MMWR Morb Mortal Wkly Rep 2010; 59:1393–9. [PubMed] [Google Scholar]
- 3.China’s all-out fight against polio. http://www.polioeradication.org/Mediaroom/Newsstories/Newsstories2011/tabid/408/iid/173/Default.aspx. Accessed 24 July 2013.
- 4.Patel MK, Konde MK, Didi-Ngossaki BH, et al. An outbreak of wild poliovirus in the Republic of Congo, 2010–2011. Clin Infect Dis 2012; 55:1291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.African Region: Namibia statistics summary (2002 - present). http://apps.who.int/gho/data/view.country.14400. Accessed 19 July 2013.
- 6.World DataBank. http://databank.worldbank.org/data/home.aspx. Accessed 22 July 2013.
- 7.WHO UNICEF estimates time series for Namibia (NAM). http://apps.who.int/immunization_monitoring/globalsummary/estimates?c=NAM. Accessed 30 July 2013.
- 8.Ministry of Health and Social Services (MOHSS). Namibia Demographic and Health Survey 1992, 1993. [Google Scholar]
- 9.Ministry of Health and Social Services (MOHSS). Namibia Demographic and Health Survey 2000 - Final report, 2003. [Google Scholar]
- 10.Ministry of Health and Social Services (MOHSS). Namibia Demographic and Health Survey 2006–07 - Final Report, 2008. [Google Scholar]
- 11.Biellik RJ, Allies T, Woodfill CJ, Lobanov A. Polio outbreaks in Namibia, 1993–1995: lessons learned. J Infect Dis 1997; 175(suppl 1): S30–6. [DOI] [PubMed] [Google Scholar]
- 12.van Niekerk AB, Vries JB, Baard J, Schoub BD, Chezzi C, Blackburn NK. Outbreak of paralytic poliomyelitis in Namibia. Lancet 1994; 344: 661–4. [DOI] [PubMed] [Google Scholar]
- 13.Advisory Committee on Polio Eradication–standing recommendations for responding to circulating polioviruses in polio-free areas. Wkly Epidemiol Rec 2005; 80:330–1. [PubMed] [Google Scholar]
- 14.World Health Organization. Polio laboratory manual. 4th ed. Vol. WHO/IVB/04.10. Geneva: World Health Organization, 2004. [Google Scholar]
- 15.Liu HM, Zheng DP, Zhang LB, Oberste MS, Pallansch MA, KewOM. Molecular evolution of a type 1 wild-vaccine poliovirus recombinant during widespread circulation in China. J Virol 2000; 74: 11153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilpatrick DR, Iber JC, Chen Q, et al. Poliovirus serotype-specific VP1 sequencing primers. J Virol Methods 2011; 174:128–30. [DOI] [PubMed] [Google Scholar]
- 17.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 2007; 24:1596–9. [DOI] [PubMed] [Google Scholar]
- 18.Kimura M A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 1980; 16:111–20. [DOI] [PubMed] [Google Scholar]
- 19.Jorba J, Campagnoli R, De L, Kew O. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J Virol 2008; 82:4429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Expanded programme on immunization. Poliomyelitis outbreak. Wkly Epidemiol Rec 1992; 67:341–4. [PubMed] [Google Scholar]
- 21.Prevots DR, Ciofi degli Atti ML, Sallabanda A, et al. Outbreak of paralytic poliomyelitis in Albania, 1996: high attack rate among adults and apparent interruption of transmission following nationwide mass vaccination. Clin Infect Dis 1998; 26:419–25. [DOI] [PubMed] [Google Scholar]
- 22.Khetsuriani N, Pallansch MA, Jabirov S, et al. Population immunity to polioviruses in the context of a large-scale wild poliovirus type 1 outbreak in Tajikistan, 2010. Vaccine 2013; 31:4911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tebbens RJ, Pallansch MA, Kew OM, et al. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal 2006; 26:1471–505. [DOI] [PubMed] [Google Scholar]
- 24.Estivariz CF, Watkins MA, Handoko D, et al. A large vaccine-derived poliovirus outbreak on Madura Island–Indonesia, 2005. J Infect Dis 2008; 197:347–54. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins HE, Aylward RB, Gasasira A, et al. Implications of a circulating vaccine-derived poliovirus in Nigeria. N Engl J Med 2010; 362: 2360–9. [DOI] [PubMed] [Google Scholar]
- 26.Cain D, Pare V, Kalichman SC, et al. HIV risks associated with patronizing alcohol serving establishments in South African Townships, Cape Town. Prev Sci 2012; 13:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nichols BE, Nkalamo D, Whitcomb BW. Density of drinking establishments and HIV prevalence in a migrant town in Namibia. AIDS Behav 2012; 16:829–34. [DOI] [PubMed] [Google Scholar]
- 28.Munch Z, Van Lill SW, Booysen CN, Zietsman HL, Enarson DA, Beyers N. Tuberculosis transmission patterns in a high-incidence area: a spatial analysis. Int J Tuberc Lung Dis 2003; 7:271–7. [PubMed] [Google Scholar]
- 29.Kalichman SC, Simbayi LC, Vermaak R, Jooste S, Cain D. HIV/AIDS risks among men and women who drink at informal alcohol serving establishments (Shebeens) in Cape Town, South Africa. Prev Sci 2008; 9:55–62. [DOI] [PubMed] [Google Scholar]