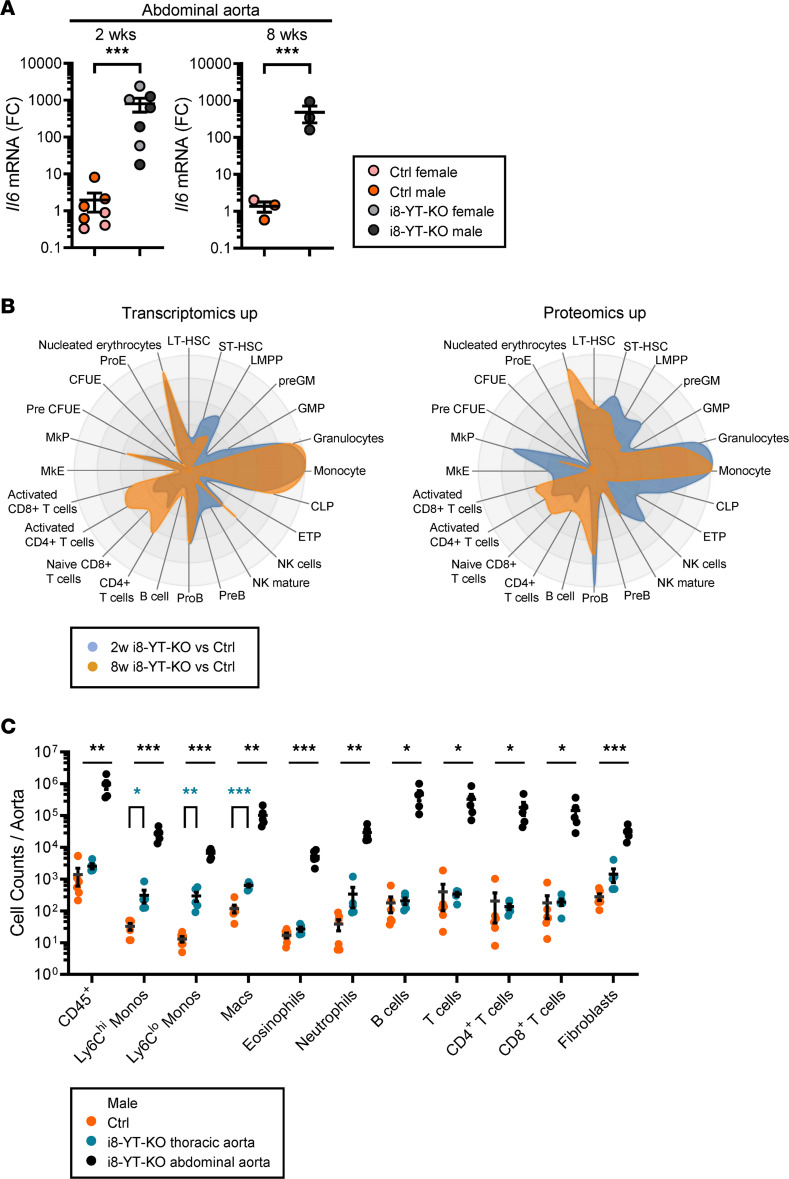
Figure 9. Aortic inflammation in i8-YT-KO mice involves induction of proinflammatory mediators and infiltration of several immune cell populations.
RNA sequencing indicated upregulation of numerous inflammatory mediators in the aorta. (A) RT-qPCR assays for the proinflammatory cytokine interleukin 6 (Il6) confirmed upregulation in the abdominal aorta (2 weeks: n = 7; 8 weeks: n = 3). FC, fold change. (B) Upregulated transcripts and proteins were next used to predict distribution of bone marrow–derived inflammatory cells using CellRadar. Irrespective of the data set, monocytes/macrophages and granulocytes were predicted to reside in knockout (i8-YT-KO) aortae. (C) To directly measure infiltration of immune cells, cells were isolated from the aorta (8 weeks) and separated by flow cytometry. We assayed the thoracic (blue) and abdominal (black) aortae separately in i8-YT-KO mice, and compared cell counts with those of the whole aorta in control (Ctrl) mice (orange). All immune cell populations were increased in the abdominal aorta, whereas only monocytes and macrophages were significantly increased in thoracic aorta. LT-HSC, long-term hematopoietic stem cells; ST-HSC, short-term hematopoietic stem cells; LMPP, lympho-myeloid primed progenitor; GM, granulocyte-macrophage; GMP, granulocyte-monocyte progenitor; CLP, common lymphoid progenitor; ETP, early T cell precursor; NK, natural killer; MkE, megakaryocyte/erythroid; MkP, megakaryocyte progenitor; CFUE, colony-forming unit-erythroid; ProE, pro-erythrocyte. *P < 0.05; **P < 0.01; ***P < 0.001 by 2-tailed Student’s t test (A and C).