Figure 2. Spatial distribution of human cells in humanized livers.
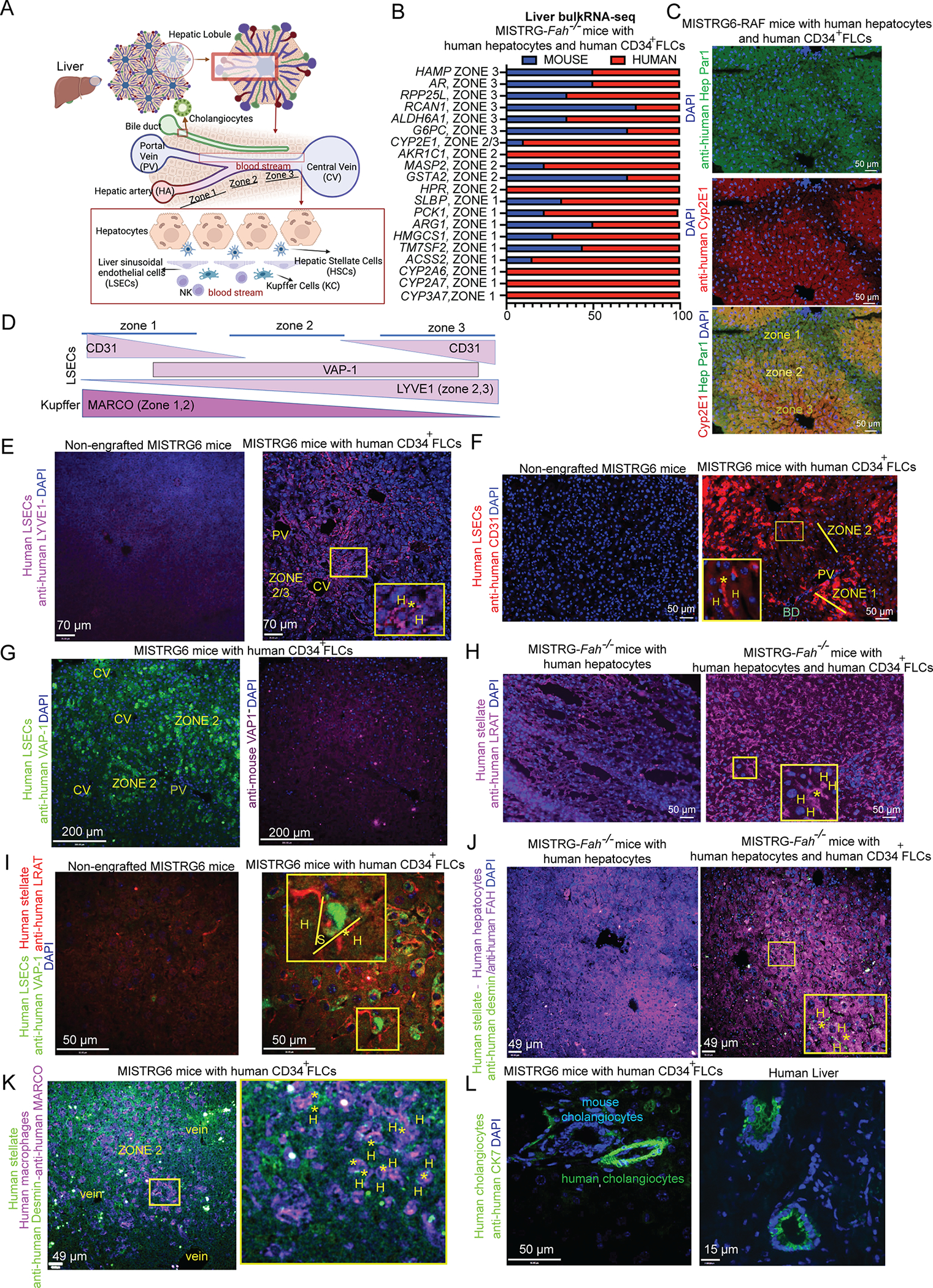
(A) Liver architecture and organization of liver cell types in the hepatic lobule. Cartoon was made using Biorender.
(B) Bulk RNA-sequencing in the liver of humanized MISTRG-Fah−/− mice. Relative abundance of mouse and human orthologous hepatocyte predominant genes.
(C) Immunofluorescence for human Cyp2E1 (zone 2, 3 protein) and human Hep Par in humanized MISTRG6-RAF mice. The antigen for Hep Par 1 antibody is the urea cycle enzyme CPS1 (zone 1, 2 protein).
(D) Expression of LSEC and Kupffer cell markers in different zones of the hepatic lobule, based upon the human protein atlas and literature24.
(E-G) Human-specific LSEC markers (LYVE-1, VAP-1, CD31) and their expression across liver zones in MISTRG6 mice engrafted with human CD34+ FLCs or non-engrafted controls. Anti-human CD31 may stain a few human macrophages. Here it is used to validate that human CD31 is not expressed in zone 2 cells. (H) Immunostaining against human LRAT
(I-K) Immunostainings for human LRAT, Desmin, FAH, VAP-1 and MARCO. Examples of the human cell types of interest, having the proper localization between hepatocytes (H) in sinusoids (S), are indicated with an asterisk (*).
(L) Immunostaining for human CK7 in humanized liver and healthy human liver sections.
All mice were analyzed 12 weeks after human CD34+ FLC (E-G, I, K-L) and human hepatocyte transplantation (C, H, J). Mice that were not engrafted with human CD34+ FLCs served as controls for the human specificity of antibodies. See also Figure S5.
