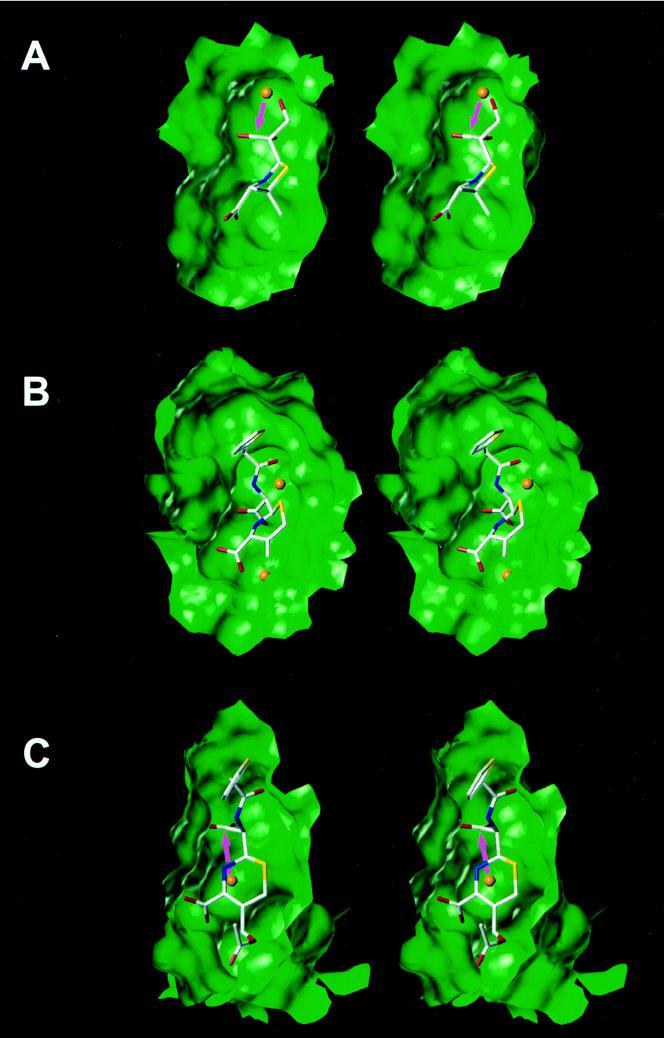
FIG. 5.
Views of the crystal structure of the acyl enzyme intermediate for 6α-(hydroxymethyl)penicillanate in the class A TEM-1 β-lactamase (A), the crystal structure of the dd-peptidase–transpeptidase from Streptomyces sp. strain R61 modified by cephalothin (the C3 substituent is eliminated because of the longevity of the species) (B), and the energy-minimized structure of the acyl enzyme intermediate for cephalothin in the active site of the class C β-lactamase from E. cloacae P99 (the C3 substituent is not eliminated because of the fleeting existence of the species) (C). The active-site cavities are shown as Connolly water-accessible surfaces. Water molecules are represented as orange spheres.