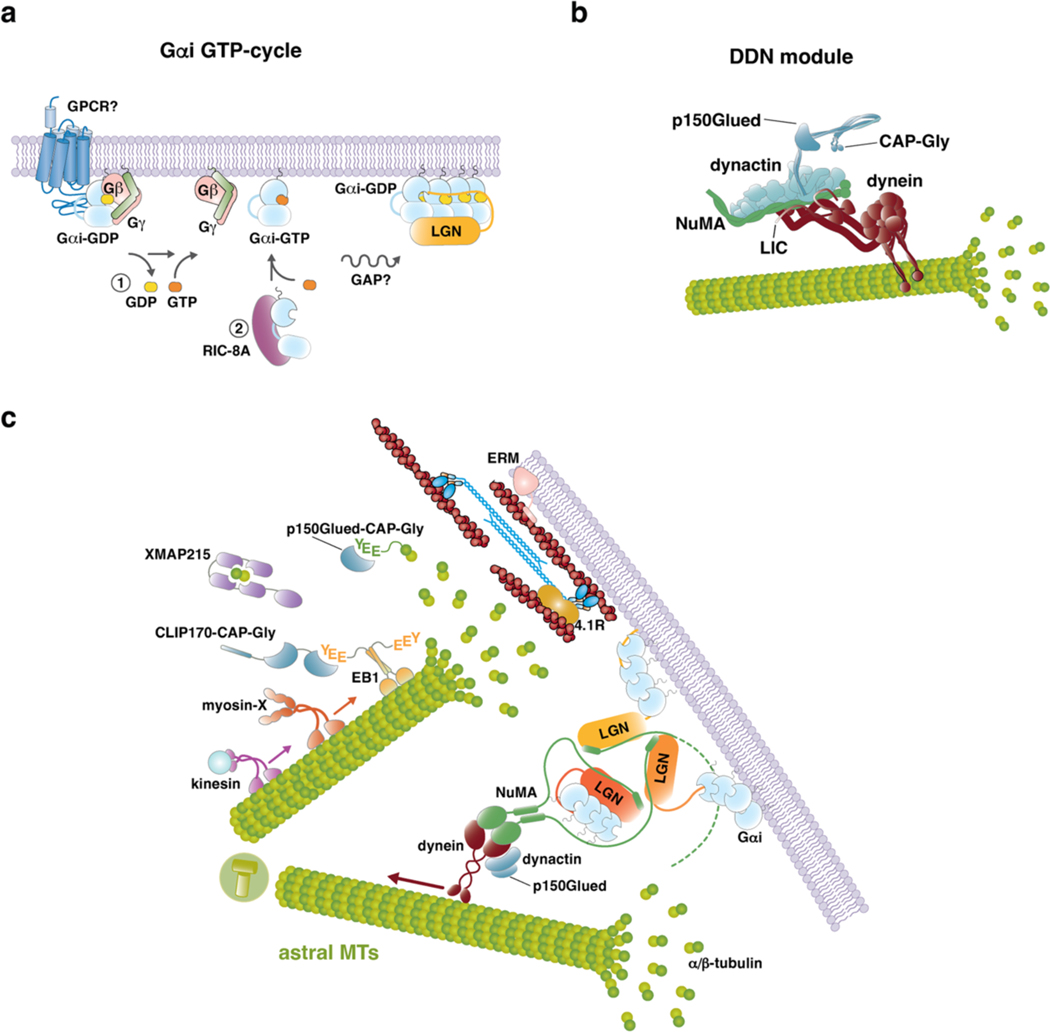
Figure 2. Working principles of cortical force generators.
a) Cartoon representation of the molecular players implicated in spindle positioning at the mitotic cortex. The force generating machines exerting traction forces on astral microtubules are organized on the dynein–dynactin–NuMA complexes, targeted to the membrane by Gαi–LGN via NuMA. Gαi-GDP accumulates at caveolin-rich membrane patches. The N-terminal portion of NuMA interacts with dynein, while the C-terminal region contacts LGN. A number of microtubule plus-TIPs binders modulate the dynamic instability of astral microtubules near the cortex, this way contributing to spindle positioning. These include: EB1, that binds with the C-terminal EEY motif to CAP-Gly containing proteins such as CLIP170 and p150Glued; XMAP215, that uses its four TOG domains [G] to assist tubulin incorporation into microtubules and stabilize the microtubule lattice at the plus ends; kinesins, such as kinesin-1. In addition, myosin X provides a physical link between astral microtubules and the cortical actin cytoskeleton, and generates pulling forces that cooperate with those produced by dynein–dynactin. Astral microtubule ends interact with the actin cortex also through mitotic interactor and substrate of PLK1 (MISP), which binds EB1 and p150Glued. The stiffness of the mitotic actomyosin cortex, needed to provide a rigid scaffold counterbalancing microtubule pulling forces exerted by dynein to position the spindle, is regulated by 4.1R proteins, likely associating to NuMA itself, and by ERM (Ezrin-Radixin-Moesin) proteins tethering F-actin to the plasma membrane b) Molecular events promoting cortical recruitment of dynein–dynactin force generators in metaphase. Top: In the unliganded form, LGN is kept inhibited by intra-molecular interactions between the N-terminal Tetratrico-Peptide-Repeat (TPR) domain and the C-terminal GoLoco region. Cooperative binding of the four GoLoco motifs to Gαi-GDP induces a conformational change allowing binding of LGN to NuMA C-terminus. Bottom: Structural evidence accumulated over the years documented the conformational rearrangement of LGN upon binding to Gαi and NuMA. In the closed conformation, the TPR domain of LGN folded as a helical cradle is occupied by the GoLoco helices, in purple (PDB-ID 4JHR). Binding of LGN to Gαi-GDP displaces the GoLoco motif from the TPR domain, that can form donut-shaped hetero-hexamers with the C-terminal portion of NuMA (PDB-ID 6HC2). Full-length NuMA dimerizes via its coiled-coil central region. The combination of the 3:3 stoichiometry observed in the LGN-TPR–NuMA-C-terminus donut-shaped structures with the dimeric nature of full-length NuMA implies that each donut connects at least two NuMA dimers — depicted by the dotted line of one of the NuMA chains departing from the hexamer — this way promoting the formation of LGN–NuMA–dynein cortical networks c) Gαi GTP-cycle associated with the assembly of Gαi–LGN–NuMA cortical complexes. In interphase, GDP-loaded Gαi forms hetero-trimeric G-protein complexes with Gβγ. To transfer Gαi from Gβγ to LGN, one possibility could be that, at mitotic entry, a localized pool of Gαi dissociates from Gβγ, possibly assisted by the GEF activity of a G-Protein-Coupled Receptor (GPCR), and generates a population of GTP-loaded Gαi molecules that, upon GTP hydrolysis, can bind and target LGN to the cortex by direct association with the four LGN-GoLoco motifs (path labelled 1 in the schematic). Alternatively (or in addition), recent evidence points at the chaperone-like activity of the scaffold RIC-8A to assist Gαi folding and GTP-loading, this way creating a GTP-bound pool of Gαi available for LGN after GTP hydrolysis (path labelled 2 in the schematic). c) Organizational principles of the dynein–dynactin–NuMA module responsible for the generation of microtubule-pulling forces orienting the spindle. Dynein is a multi-subunit complex comprising two-copies of six different chains, including a microtubule-binding stalk and an AAA+ ATPase domain. In mitosis, NuMA binds the light intermediate chain (LIC) subunit of dynein, likely sandwiching between dynein and the dynactin complex, which has been shown to confer processivity to the motor activity of dynein. Dynactin itself consists of several subunits, including a p150Glued subunit encompassing a CAP-Gly domain.