Abstract
Background:
The aim of this study was to systematically evaluate the prognostic role of platelet lymphocyte ratio (PLR) in patients with melanoma through performing a meta-analysis.
Methods:
PubMed, EMBASE, Web of Science, and China National Knowledge Infrastructure were searched for potential studies. The basic characteristics and relevant data were extracted. Hazard ratios with 95% confidence intervals (CIs) were pooled to evaluate the prognostic role of PLR in patients with melanoma.
Results:
Ten studies enrolling 2422 patients were included. The pooled hazard ratios of higher PLR for overall survival and progression-free survival in melanoma were 1.70 (95% CI, 1.22–2.37) and 1.65 (95% CI, 1.10–2.47), respectively. Sensitivity analysis and subgroup analyses were also performed. No significant publication bias was observed.
Conclusion:
Our results showed that higher PLR was associated with poorer overall survival and progression-free survival in patients with melanoma. These findings may help to determine the prognosis and explore future novel therapies based on modulating inflammation and immune responses in melanoma.
Keywords: melanoma, platelet lymphocyte ratio, prognosis, survival
1. Introduction
Melanoma accounts for about 287,723 newly diagnosed cancer cases and 60,712 cancer deaths worldwide in 2018.[1] It is estimated that melanoma is the most rapidly increasing cancer in men and the second most rapidly increasing cancer in women in the United States.[2] Melanoma in most patients are localized and could be treated by surgical resection. For patients with advanced-stage, immunotherapy, novel targeted therapy, and stereotactic radiosurgery are promising therapies.[2,3] The prognosis of melanoma in the early stage is good, but the prognosis of melanoma in the advanced stage is still poor, with a 10-year OS rate of only 10% to 15%.[4] Therefore, it is essential to identify effective biomarkers for the prognosis and management of melanoma.
In recent years, accumulating evidence demonstrated that inflammation played a crucial role in cancer growth and metastasis, and could be prognostic markers in a variety of cancers.[5–8] Several inflammatory markers in blood have been proposed prognostic markers in melanoma, such as neutrophil–lymphocyte ratio, platelet lymphocyte ratio (PLR), and C-reactive protein.[9–11] In recent years, the prognostic value of PLR in melanoma is controversial. Some researchers showed that higher PLR was related to poorer survival in melanoma.[12,13] However, some researchers found that PLR could not be a prognostic marker in melanoma.[14,15] Therefore, this meta-analysis aimed to systematically evaluate the prognostic value of PLR in patients with melanoma.
2. Methods
2.1. Search strategy
Since this is a meta-analysis, ethical approval was waived. This meta-analysis was conducted according to the developed guidelines for performing meta-analyses.[16] The following databases were searched for eligible studies: PubMed, EMBASE, Web of Science, and China National Knowledge Infrastructure (last search on May 30, 2020). The keywords included: melanoma, platelet lymphocyte ratio/PLR, and prognosis/survival/outcome/prognostic. References of the relevant articles were also screened for additional studies. Languages were restricted to English and Chinese.
2.2. Study selection
Two authors independently performed the study selection and disagreements were resolved by discussion. Titles and abstracts of the papers were reviewed first and then the potential studies were screened in full text. Inclusion criteria included: (1) patients were diagnosed with melanoma by pathological examination; (2) platelet count and lymphocyte count of the patients were measured and PLR was calculated; (3) patients were followed up for survival analyses; and (4) enough data was reported to measure the prognostic value of PLR in patients with melanoma. Case reports, reviews, letters, unrelated articles, and studies without enough data were excluded.
2.3. Data extraction
Data were also extracted independently by 2 authors, and disagreements were resolved by consensus. The primary data were hazard ratio (HR) for overall survival (OS) and progression-free survival (PFS) with 95% confidence interval (CI), or survival curves with P values. HRs from multivariate analyses were extracted over those from univariate analyses. The basic characteristics of the studies included the first author, publication year, country, study type, number of patients, sex, age, tumor type, metastatic status, tumor stage, treatment, and cutoff value of PLR.
2.4. Quality assessment of studies
The quality of the studies was assessed by Newcastle–Ottawa Scale (NOS), including study population, comparability, and outcome.[17] The score ranges from 0 to 9, and 7 to 9 points indicate high-quality studies.
2.5. Statistical analysis
The prognostic value of PLR in patients with melanoma was assessed by outlining forest plots. Log HR and variance calculated by HR with 95% CIs were used for aggregation, and P < .05 was regarded statistically significant. Heterogeneity across the studies was assessed, and I2 > 50% or P < .10 indicates significant heterogeneity. Since heterogeneity across studies existed due to the different characteristics of patients, random effect models were always used in combining the data. If heterogeneity was significant, sensitivity analysis was performed to assess the contribution of each study to heterogeneity by excluding the studies one by one. Subgroup analyses were also performed according to country, tumor type, metastatic status, treatment, and cutoff value of PLR. Publication bias was assessed by Begg test and P < .05 indicated significant publication bias. All the above statistical analyses were performed by STATA 11.0 (STATA Corporation, College Station, TX).
3. Results
3.1. Literature research
The initial literature search retrieved 205 studies. No additional studies were identified through other sources. After removing the duplicates, 169 studies were screened by titles and abstracts. Among them, 148 studies were excluded according to the inclusion and exclusion criteria. The rest 21 studies were evaluated in full text and 11 studies were excluded due to unrelated, lacking data, or other reasons. Finally, 10 studies[11–15,18–22] met the inclusion criteria and were included. The study selection process was shown in Figure 1.
Figure 1.
Selection process of the studies.
3.2. Study characteristics
The basic characteristics of the included studies were shown in Table 1. The studies were published in the latest 4 years and were from 5 different countries. The studies were all retrospective clinical studies. A total of 2422 patients were included. The tumor type included cutaneous, acral, mucosal, and mixed types. The metastatic status included metastatic, non-metastatic, and mixed. Treatments included immunotherapy, chemotherapy, surgical resection, and mixed treatments. The cutoff values of PLR varied from 99 to 206. The survival outcomes included OS and PFS. The NOS score was 7 or more in 9 studies and 6 in 1 study.
Table 1.
Characteristics of the included studies.
| Author | Year | Country | Study type | N | Female | Male | Median age | Tumor type | Metastatic status | Stage | Treatment | Cutoff value | Outcome | NOS score |
| Khoja | 2016 | Canada | Retrospective | 183 | 68 | 115 | 58 (24–89) | Cutaneous | Metastatic | IV | Immunotherapy | 182 | OS/PFS | 8 |
| Yu | 2017 | China | Retrospective | 226 | NR | NR | NR | Acral | Metastatic | IV | Immunotherapy | 129 | OS/PFS | 7 |
| Minowa | 2018 | Japan | Retrospective | 21 | 10 | 11 | 74 (34–91) | Mixed | Metastatic | IV | Immunotherapy | 159 | OS | 7 |
| Qi | 2018 | China | Retrospective | 140 | 71 | 69 | 56.4 (22–81) | Mixed | Mixed | I–IV | Mixed | 120.15 | OS | 8 |
| Wade | 2018 | UK | Retrospective | 1351 | 673 | 678 | NR | Cutaneous | Non-metastatic | I–III | Surgical resection | 100 | OS/PFS | 9 |
| Wang Yixi | 2019 | China | Retrospective | 40 | 26 | 14 | 58 | Mucosal | Mixed | I–IV | Mixed | 118.7 | OS/PFS | 8 |
| Yang | 2019 | China | Retrospective | 55 | 32 | 23 | 58 (38–75) | Mixed | Metastatic | III–IV | Chemotherapy | 206 | PFS | 7 |
| Martins | 2019 | Portugal | Retrospective | 83 | 42 | 41 | 65.5 | Mixed | Metastatic | IV | Immunotherapy | 180 | OS/PFS | 6 |
| Cao | 2018 | China | Retrospective | 120 | 53 | 67 | 57 (19–86) | Cutaneous | Non-metastatic | I–III | Surgical resection | 99 | OS | 7 |
| Wang Yao | 2019 | China | Retrospective | 223 | 87 | 136 | 55.6 (18–85) | Acral | Non-metastatic | I–III | Surgical resection | 113.6 | OS | 7 |
N = number of patients, NOS = Newcastle–Ottawa Scale, NR = not reported, OS = overall survival, PFS = progression-free survival.
3.3. Overall analysis
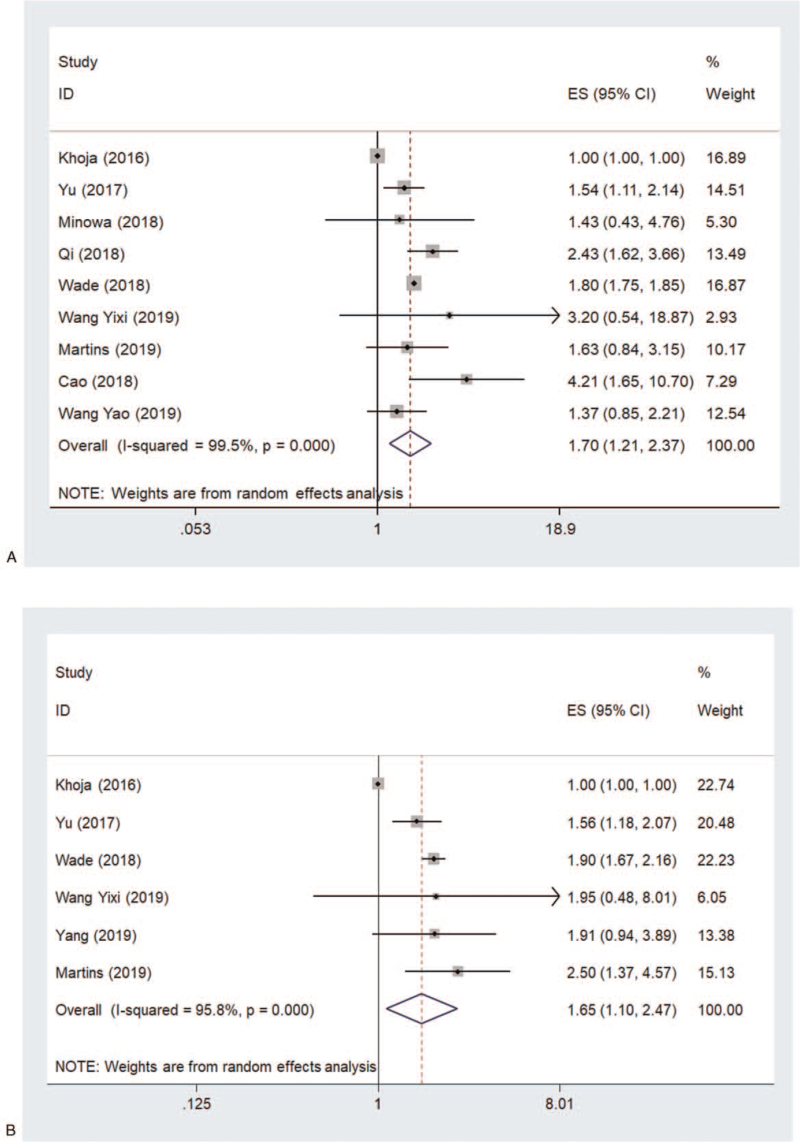
Nine of the 10 studies assessed the association between PLR level and OS. The pooled HR of higher PLR for OS was 1.70 (95% CI, 1.22–2.37) (Fig. 2A). Significant heterogeneity was observed across the studies (I2 = 99.5%, P < .001). Sensitivity analysis revealed that the study by Khoja et al[18] was a major contributor to heterogeneity. After excluding this study, the heterogeneity dropped sharply to 12.2% and the pooled HR remained significant (1.80, 95% CI, 1.60–2.03).
Figure 2.
Pooled HR of higher PLR for (A) OS and (B) PFS in patients with melanoma. HR = hazard ratio, OS = overall survival, PFS = progression-free survival, PLR = platelet lymphocyte ratio.
Six of the 10 studies assessed the association between PLR level and PFS. The pooled HR of higher PLR for PFS was 1.65 (95% CI, 1.10-2.47) (Fig. 2B). Significant heterogeneity was also observed across the studies (I2 = 95.8%, P < .001). Sensitivity analysis revealed that the study by Khoja et al[18] was also a major contributor to heterogeneity. After excluding this study, the heterogeneity became 0.0% and the pooled HR remained significant (1.86, 95% CI, 1.66–2.08).
3.4. Subgroup analysis
Due to the major contribution to the heterogeneity of the study by Khoja et al,[18] it was excluded in subgroup analysis.
3.4.1. Country
The pooled HRs of higher PLR for OS in Asian and non-Asian patients were 1.89 (95% CI, 1.39–2.58) and 1.80 (95% CI, 1.75–1.85), respectively. The pooled HRs of higher PLR for PFS in Asian and non-Asian patients were 1.62 (95% CI, 1.25–2.09) and 1.92 (95% CI, 1.70–2.18), respectively.
3.4.2. Tumor type
The pooled HRs of higher PLR for OS in mixed, cutaneous, and acral groups were 2.11 (95% CI, 1.51–2.94), 2.41 (95% CI, 1.09–5.30), and 1.49 (95% CI, 1.13–1.95), respectively. One study examined the mucosal type and the HR for OS was 3.20 (95% CI, 0.54–18.88). The pooled HR of higher PLR for PFS in the mixed group was 2.23 (95% CI, 1.41–3.54). The number of studies in the cutaneous, acral, and mucosal group was 1, and the HRs for PFS were 1.90 (95% CI, 1.70-2.20), 1.56 (95% CI, 1.18–2.07), and 1.95 (95% CI, 0.48–8.02), respectively.
3.4.3. Metastatic status
The pooled HRs of higher PLR for OS in mixed, metastatic, and non-metastatic groups were 2.46 (95% CI, 1.66–3.67), 1.55 (95% CI, 1.16–2.06), and 1.84 (95% CI, 1.28–2.64), respectively. The pooled HR of higher PLR for PFS in the metastatic group was 1.72 (95% CI, 1.35–2.20). The number of studies in the mixed and non-metastatic group was 1, and the HRs for PFS were 1.95 (95% CI, 0.48–8.02) and 1.90 (95% CI, 1.70–2.20), respectively.
3.4.4. Treatment
The pooled HRs of higher PLR for OS in mixed, immunotherapy, and surgical resection groups were 2.46 (95% CI, 1.66–3.67), 1.55 (95% CI, 1.16–2.06), and 1.84 (95% CI, 1.28–2.64), respectively. The pooled HR of higher PLR for PFS in the immunotherapy group was1.83 (95% CI, 1.18–2.82). The number of studies in the mixed, chemotherapy, and surgical resection group was 1, and the HRs for PFS were 1.95 (95% CI, 0.48–8.02), 1.91 (95% CI, 0.94–3.90), and 1.90 (95% CI, 1.70–2.20), respectively.
3.4.5. Cutoff value of PLR
The pooled HRs of higher PLR for OS in cutoff <120 and cutoff >120 groups were 1.85 (95% CI, 1.36–2.53) and 1.80 (95% CI, 1.41–2.30), respectively. The pooled HRs of higher PLR for PFS in cutoff <120 and cutoff >120 groups were 1.90 (95% CI, 1.67–2.16) and 1.72 (95% CI, 1.35–2.20), respectively.
All the meta-analyses results were summarized in Table 2.
Table 2.
Summary of meta-analysis results.
| Variables | No. of studies | Pooled HR (95% CI) | P value | Heterogeneity (I2, P) |
| OS | 9 | 1.70 (1.22–2.37) | .002 | 99.5%, <.001 |
| Country | ||||
| Asian | 6 | 1.89 (1.39–2.58) | <.001 | 36.5%, .163 |
| Non-Asian | 2 | 1.80 (1.75–1.85) | <.001 | 0.0%, .762 |
| Tumor type | ||||
| Mixed | 3 | 2.11 (1.51–2.94) | <.001 | 0.0%, .482 |
| Cutaneous | 2 | 2.41 (1.09–5.30) | .029 | 68.5%, <.075 |
| Acral | 2 | 1.49 (1.13–1.95) | .004 | 0.0%, .693 |
| Mucosal | 1 | 3.20 (0.54–18.88) | — | — |
| Metastatic status | ||||
| Mixed | 2 | 2.46 (1.66–3.67) | <.001 | 0.0%, .768 |
| Metastatic | 3 | 1.55 (1.16–2.06) | .003 | 0.0%, .981 |
| Non-metastatic | 3 | 1.84 (1.28–2.64) | .001 | 54.7%, .110 |
| Treatment | ||||
| Mixed | 2 | 2.46 (1.66–3.67) | <.001 | 0.0%, .768 |
| Immunotherapy | 3 | 1.55 (1.16–2.06) | .003 | 0.0%, .981 |
| Surgical resection | 3 | 1.84 (1.28–2.64) | .001 | 54.7%, .110 |
| Cutoff value of PLR | ||||
| Cutoff <120 | 4 | 1.85 (1.36–2.53) | <.001 | 37.7%, .186 |
| Cutoff >120 | 4 | 1.80 (1.41–2.30) | <.001 | 5.0%, .368 |
| PFS | 6 | 1.65 (1.10–2.47) | .016 | 95.8%, <.001 |
| Country | ||||
| Asian | 3 | 1.62 (1.25–2.09) | <.001 | 0.0%, .844 |
| Non-Asian | 2 | 1.92 (1.70–2.18) | <.001 | 0.0%, .384 |
| Tumor type | ||||
| Mixed | 2 | 2.23 (1.41–3.54) | .001 | 0.0%, .572 |
| Cutaneous | 1 | 1.90 (1.70–2.20) | — | — |
| Acral | 1 | 1.56 (1.18–2.07) | — | — |
| Mucosal | 1 | 1.95 (0.48–8.02) | — | — |
| Metastatic status | ||||
| Mixed | 1 | 1.95 (0.48–8.02) | — | — |
| Metastatic | 3 | 1.72 (1.35–2.20) | <.001 | 0.4%, .366 |
| Non-metastatic | 1 | 1.90 (1.70–2.20) | — | — |
| Treatment | ||||
| Mixed | 1 | 1.95 (0.48–8.02) | — | — |
| Immunotherapy | 2 | 1.83 (1.18–2.82) | .007 | 47.8%, .166 |
| Chemotherapy | 1 | 1.91 (0.94–3.90) | — | — |
| Surgical resection | 1 | 1.90 (1.70–2.20) | — | — |
| Cutoff value of PLR | ||||
| Cutoff <120 | 2 | 1.90 (1.67–2.16) | <.001 | 0.0%, .970 |
| Cutoff >120 | 3 | 1.72 (1.35–2.20) | <.001 | 0.4%, .366 |
CI = confidence interval, HR = hazard ratio, OS = overall survival, PFS = progression-free survival, PLR = platelet lymphocyte ratio.
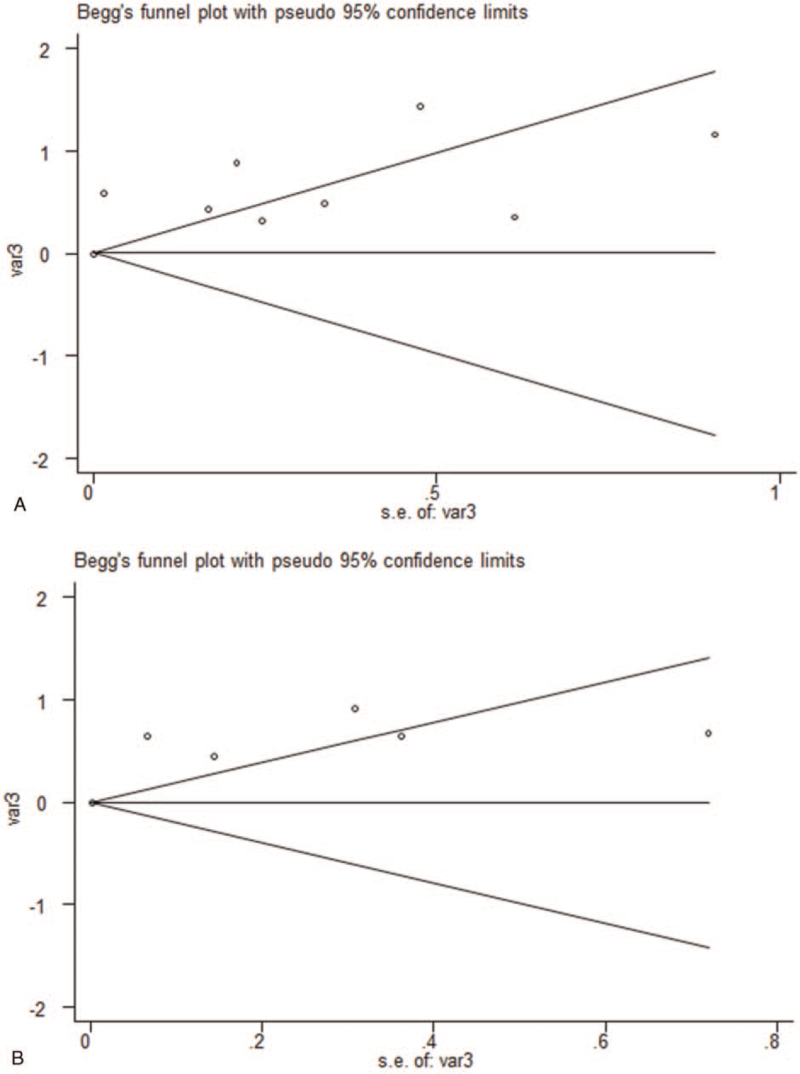
3.5. Publication bias
No significant publication bias was observed in the meta-analysis. The Begg plots of publication bias of the 9 studies for OS (P = .602) and 6 studies for PFS (P = .452) were shown in Figure 3A and B.
Figure 3.
The Begg plots of publication bias of the studies for (A) OS and (B) PFS. OS = overall survival, PFS = progression-free survival.
4. Discussion
This study aimed to evaluate the prognostic value of PLR in patients with melanoma. A meta-analysis was performed to summarize the existing evidence. A total of 10 studies were included. The results demonstrated that higher PLR was associated with poorer OS and PFS in patients with melanoma.
Subgroup analyses were performed to further explore the role of PLR in patients with melanoma. As to different countries, higher PLR was found to be associated with worse OS and PFS in both Asian and non-Asian patients, implying the prognostic value of PLR in different populations. As to different tumor types, PLR was a prognostic marker for OS and PFS in mixed, cutaneous, and acral groups, except for mucosal type. The reason might be that only 1 study investigated mucosal type and more studies are needed. As to metastatic status, PLR was a prognostic marker for OS in all groups but was not a prognostic marker for PFS in the mixed group. The reason might also be that only 1 study was in the mixed group for PFS. As to different treatments, PLR was a prognostic marker for OS in all groups but was not a prognostic marker for PFS in the mixed group and chemotherapy group. More studies are also needed since only 1 study was in the mixed group and chemotherapy group for PFS. As to different cutoff values of PLR, PLR was a prognostic marker for OS, and PFS in both groups. Thus, more studies are warranted to verify the results in the subgroup analyses.
Inflammation and immune responses in the tumor microenvironment play an important role in cancer development, such as proliferation, angiogenesis, and metastasis.[23] Platelet and lymphocyte are important indicators of systemic inflammation. Platelets could secrete various cytokines to support tumor growth, protect tumor from apoptosis, and promote tumor metastasis, such as platelet-derived growth factor and interleukin-6.[24,25] In contrast, lymphocytes usually play an essential role in T cell-mediated antitumor response. Lymphocytes can suppress the tumor through the induction of cytotoxic cell death and the suppression of tumor proliferation.[26,27] Taken together, higher PLR, the result of the increase of platelets and/or the decrease of lymphocytes, indicates a low antitumor effect and predicts poor prognosis.
Apart from melanoma, many studies assessed the prognostic value of PLR in human cancers through meta-analyses. Zhang et al found that an elevated pre-treatment PLR was a prognostic factor for poor OS and disease-free survival (DFS) in gastric cancer patients.[28] Another study demonstrated that pre-treatment PLR could serve as a prognostic biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors.[29] Bardash et al demonstrated that an elevated PLR was significantly associated with poorer OS and disease-specific survival.[30] In consistent with the above research, our meta-analysis also proved that higher PLR was a predictor of worse OS and PFS.
There are several limitations in our meta-analysis. Firstly, the number of studies in this meta-analysis was limited. Therefore, the pooled results, especially the conclusions in the subgroup analyses, should be treated with caution, and more studies are needed. Secondly, the basic characteristics of the studies were different, such as tumor type, metastatic status, treatment, and cutoff values of PLR. Therefore, subgroup analyses were performed according to the characteristics. However, more studies are still warranted in the future. Besides, significant heterogeneity across the studies was found. Sensitivity analysis identified that the study by Khoja et al was a major contributor to heterogeneity. After excluding this study, the pooled HRs for OS and PFS both remained significant. Furthermore, publication bias should not be completely excluded, although no significant publication bias was observed.
5. Conclusions
In conclusion, our results showed that higher PLR was associated with poorer OS and PFS in patients with melanoma. These findings may help to determine the prognosis and explore future novel therapies based on modulating inflammation and immune responses in melanoma. However, due to the limitations of this meta-analysis, more well-designed studies are warranted to verify our results.
Author contributions
Conceptualization: Enwen Wang, Huiwen Ma.
Data curation: Enwen Wang, Hui Huang, Long Tang, Ling Tian, Liejun Yang, Sixiong Wang, Huiwen Ma.
Formal analysis: Enwen Wang, Hui Huang, Ling Tian, Sixiong Wang, Huiwen Ma.
Funding acquisition: Enwen Wang, Hui Huang.
Methodology: Long Tang, Liejun Yang.
Software: Enwen Wang, Hui Huang, Long Tang, Ling Tian, Sixiong Wang.
Supervision: Huiwen Ma.
Writing – original draft: Enwen Wang, Hui Huang, Huiwen Ma.
Writing – review & editing: Enwen Wang, Hui Huang, Long Tang, Ling Tian, Liejun Yang, Sixiong Wang, Huiwen Ma.
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, NOS = Newcastle–Ottawa Scale, OS = overall survival, PFS = progression free survival, PLR = platelet lymphocyte ratio.
How to cite this article: Wang E, Huang H, Tang L, Tian L, Yang L, Wang S, Ma H. Prognostic significance of platelet lymphocyte ratio in patients with melanoma: a meta-analysis. Medicine. 2021;100:38(e27223).
This work was supported by Chongqing Municipal Health Commission (No. 2017ZDXM026) and Chongqing Municipal Science and Technology Commission (No. cstc2019jcyj-msxmX0640).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Coit DG, Thompson JA, Albertini MR, et al. Cutaneous Melanoma, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:367–402. [DOI] [PubMed] [Google Scholar]
- [3].Richtig E. ASCO Congress 2018: melanoma treatment. Memo 2018;11:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].O’Neill CH, Scoggins CR. Melanoma. J Surg Oncol 2019;120:873–81. [DOI] [PubMed] [Google Scholar]
- [5].Ji Y, Wang H. Prognostic prediction of systemic immune-inflammation index for patients with gynecological and breast cancers: a meta-analysis. World J Surg Oncol 2020;18:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kim MR, Kim AS, Choi HI, Jung JH, Park JY, Ko HJ. Inflammatory markers for predicting overall survival in gastric cancer patients: a systematic review and meta-analysis. PloS One 2020;15:e0236445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang L, Li L, Liu J, et al. Meta-analysis of multiple hematological biomarkers as prognostic predictors of survival in bladder cancer. Medicine 2020;99:e20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang Y, Liu F, Wang Y. Evidence of the prognostic value of pretreatment systemic inflammation response index in cancer patients: a pooled analysis of 19 cohort studies. Dis Markers 2020;2020:8854267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Qi Y, Liao D, Mei D, Zhang Y, Liu Y. Elevated neutrophil-to-lymphocyte ratio is associated with poor outcomes for melanoma patients treated with PD-1 inhibitor or chemotherapy in a Chinese population. Front Oncol 2020;10:1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Iivanainen S, Ahvonen J, Knuuttila A, Tiainen S, Koivunen JP. Elevated CRP levels indicate poor progression-free and overall survival on cancer patients treated with PD-1 inhibitors. ESMO Open 2019;4:e000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Minowa T, Kato J, Hida T, et al. Prognostic role of platelet to lymphocyte and lymphocyte to monocyte ratios in advanced melanoma treated with anti-programmed death-1. Eur J Dermatol 2018;28:705–7. [DOI] [PubMed] [Google Scholar]
- [12].Qi Y, Zhang Y, Fu X, et al. Platelet-to-lymphocyte ratio in peripheral blood: a novel independent prognostic factor in patients with melanoma. Int Immunopharmacol 2018;56:143–7. [DOI] [PubMed] [Google Scholar]
- [13].Wade RG, Robinson AV, Lo MCI, et al. Baseline neutrophil-lymphocyte and platelet-lymphocyte ratios as biomarkers of survival in cutaneous melanoma: a multicenter cohort study. Ann Surg Oncol 2018;25:3341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang Y, Zhang H, Yang Y, Zhang T, Ma X. Prognostic value of peripheral inflammatory markers in preoperative mucosal melanoma: a multicenter retrospective study. Front Oncol 2019;9:995–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang Y, Ba H, Wen X, Zhang X. Relationship between preoperative platelet level and prognosis in patients with resectable acral melanoma. J Chin Oncol 2019;25:646–9. [Google Scholar]
- [16].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [18].Khoja L, Atenafu EG, Templeton A, et al. The full blood count as a biomarker of outcome and toxicity in ipilimumab-treated cutaneous metastatic melanoma. Cancer Med 2016;5:2792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yu J, Wu X, Yu H, et al. Systemic immune-inflammation index and circulating T-cell immune index predict outcomes in high-risk acral melanoma patients treated with high-dose interferon. Transl Oncol 2017;10:719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang L, Xu Y, Luo P, Chen S, Zhu H, Wang C. Baseline platelet counts and derived inflammatory biomarkers: prognostic relevance in metastatic melanoma patients receiving Endostar plus dacarbazine and cisplatin. Cancer Manag Res 2019;11:3681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Martins SL, Miguelsemedo P, Martinsbranco DA, et al. Hematological profile: a prognosis tool in melanoma patients treated with immunotherapy. J Clin Oncol 2019;37. [Google Scholar]
- [22].Cao Y, Zhang W, Du W, Wang X, Cao S. Higher preoperative platelet-to-lymphocyte ratio is a poor prognostic marker for the early stage malignant melanoma patients. Chin J Cancer Biother 2018;25:509–14. [Google Scholar]
- [23].Bald T, Quast T, Landsberg J, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 2014;507:109–13. [DOI] [PubMed] [Google Scholar]
- [24].Cho MS, Bottsford-Miller J, Vasquez HG, et al. Platelets increase the proliferation of ovarian cancer cells. Blood 2012;120:4869–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Velez J, Enciso LJ, Suarez M, et al. Platelets promote mitochondrial uncoupling and resistance to apoptosis in leukemia cells: a novel paradigm for the bone marrow microenvironment. Cancer Microenviron 2014;7:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137–48. [DOI] [PubMed] [Google Scholar]
- [27].Man YG, Stojadinovic A, Mason J, et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories. J Cancer 2013;4:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang X, Zhao W, Yu Y, et al. Clinicopathological and prognostic significance of platelet-lymphocyte ratio (PLR) in gastric cancer: an updated meta-analysis. World J Surg Oncol 2020;18:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang N, Jiang J, Tang S, Sun G. Predictive value of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in non-small cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Int Immunopharmacol 2020;85:106677. [DOI] [PubMed] [Google Scholar]
- [30].Bardash Y, Olson C, Herman W, Khaymovich J, Costantino P, Tham T. Platelet-lymphocyte ratio as a predictor of prognosis in head and neck cancer: a systematic review and meta-analysis. Oncol Res Treat 2019;42:665–77. [DOI] [PubMed] [Google Scholar]