Figure 2.
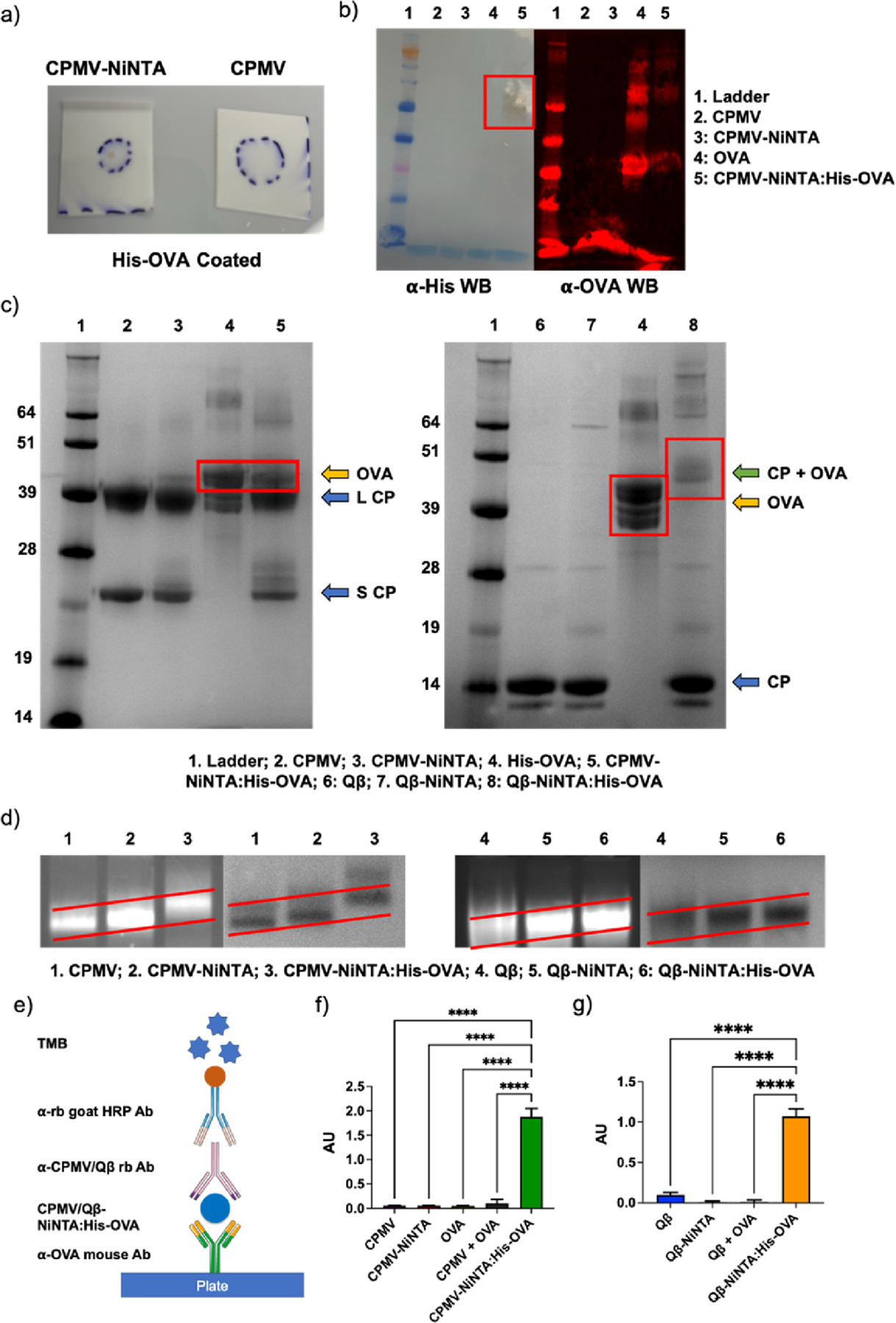
Characterization of NiNTA:His-OVA vaccine formulations. (a) DB of CPMV-NiNTA vs CPMV against His-OVA on a nitrocellulose membrane. (b) WB against His-tag (left) and OVA (right). (c) SDS-PAGE. In CPMV-NiNTA:His-OVA (left), OVA dissociates from the complex (lane 4); however, in Qβ-NiNTA:His-OVA (right), the CP and OVA remain associated (lane 8). (d) Agarose gel electrophoresis of the vaccine formulations. The increasing molecular weight is better demonstrated by the sloped red lines. Left is RNA staining, right is protein staining. (e) Schematic of ELISA. (f) CPMV-NiNTA:His-OVA ELISA and controls. (g) Qβ-NiNTA:His-OVA ELISA and controls. **** = p < 0.0001. The schematic in (e) was created using Biorender.com.
