Abstract
Pyronaridine-artesunate was recently strongly recommended in the 2022 update of the WHO Guidelines for the Treatment of Malaria, becoming the newest artemisinin-based combination therapy (ACT) for both uncomplicated Plasmodium falciparum and Plasmodium vivax malaria. Pyronaridine-artesunate, available as a tablet and paediatric granule formulations, is being adopted in regions where malaria treatment outcome is challenged by increasing chloroquine resistance. Pyronaridine is an old antimalarial agent that has been used for more than 50 years as a blood schizonticide, which exerts its antimalarial activity by interfering with the synthesis of the haemozoin pigment within the Plasmodium digestive vacuole. Pyronaridine exhibits a high blood-to-plasma distribution ratio due to its tendency to accumulate in blood cells. This feature is believed to play a crucial role in its pharmacokinetic (PK) properties and pharmacological activity. The PK characteristics of pyronaridine include rapid oral absorption, large volumes of distribution and low total body clearance, resulting in a long terminal apparent half-life. Moreover, differences in PK profiles have been observed between healthy volunteers and malaria-infected patients, indicating a potential disease-related impact on PK properties. Despite a long history, there is only limited knowledge of the clinical PK and pharmacodynamics of pyronaridine, particularly in special populations such as children and pregnant women. We here provide a comprehensive overview of the clinical pharmacology of pyronaridine in the treatment of malaria.
Introduction
History of pyronaridine
Pyronaridine is a Mannich base with potent antimalarial and potential antiviral activities. It has been used as an antimalarial agent for over 50 years against Plasmodium falciparum and Plasmodium vivax. In the 1970s, pyronaridine was first synthesized and used in China as a monotherapy regimen, under the trade name Malaridine.1,2 In the 1990s, due to the spread of MDR P. falciparum in Africa and Asia, pyronaridine attracted wider global attention and underwent extensive studies outside China.3,4 In 1996, a landmark clinical trial concluded that pyronaridine demonstrated outstanding efficacy and good tolerability in adults and children with acute uncomplicated P. falciparum malaria.5,6
In the 2000s, interest was renewed in pyronaridine as a potential partner drug in artemisinin-based combination therapies (ACTs) for the treatment of P. falciparum malaria. In short, ACTs combine a fast-acting artemisinin derivative and a longer-acting partner drug, in which the artemisinin-based drug rapidly eliminates the majority of malaria parasites and the companion drug prevents recrudescence and resistance.7 Currently, pyronaridine is formulated in a 3:1 ratio with artesunate (pyronaridine-artesunate) as a fixed-dose ACT under the brand name Pyramax®.8 Pyronaridine-artesunate is prescribed as a once-daily, 3 day therapy for the treatment of uncomplicated P. falciparum and P. vivax malaria in adults and children.9
Role of pyronaridine in the treatment of malaria
In 2012, pyronaridine-artesunate received a positive scientific opinion under the EMA Article 58 procedure and it became the first ACT registered with a stringent regulatory authority. In the same year, it was included in WHO’s list of prequalified medicines for malaria.9 In 2017, pyronaridine-artesunate was added to the WHO’s Essential Medicines List (EML) and Essential Medicines List for Children (EMLc).10 However, due to hepatotoxicity concerns, the WHO Guidelines for the Treatment of Malaria released in 2015 did not recommend the general use of pyronaridine-artesunate.11 In 2019, supported by a WHO information note, the safety restrictions on the use of pyronaridine-artesunate were no longer justified.12 In the recently updated WHO Guidelines for malaria from 2022, the use of pyronaridine-artesunate was reconfirmed with a ‘STRONG’ recommendation indicating the highest level of confidence. Currently, pyronaridine-artesunate is considered a safe and efficacious ACT for the treatment of uncomplicated malaria in adults and children weighing 5 kg and over in all malaria-endemic areas.13
Methods
The literature database PubMed was searched with the following term: (pyronaridine) and (malaria). Relevant secondary publications, such as dissertations and other publications not published in scientific journals, related to clinical pharmacology and pharmacokinetics of pyronaridine, were identified through a Google Scholar search, using the term: (pyronaridine) and ((pharmacokinetics) or (clinical pharmacology)).
Pharmacological class and physicochemical properties
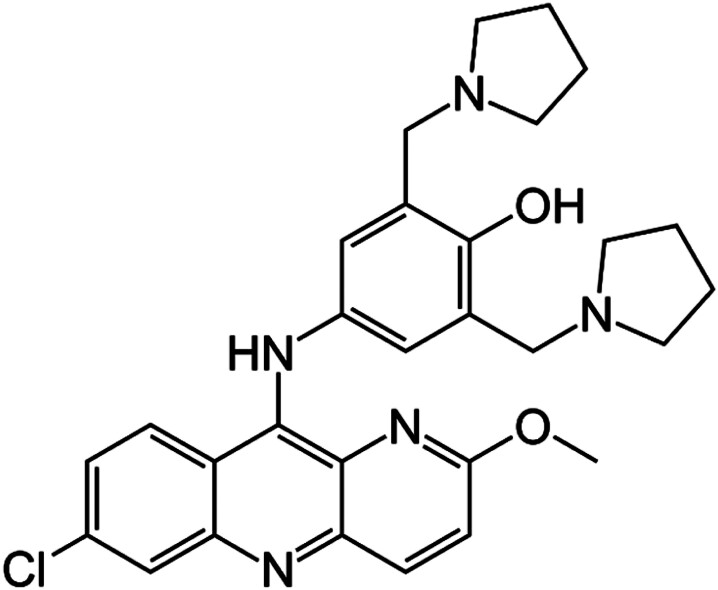
Pyronaridine is a benzo-naphthyridine derivative, with a mepacrine (9-aminoacridine) component and an additional amodiaquine-like side chain.14 The chemical name of pyronaridine is 4-[(7-chloro-2-methoxybenzo[b][1,5]naphthyridin-10-yl)amino]-2,6-bis(pyrrolidin-1-ylmethyl)phenol (Figure 1). Its empirical formula is C29H32ClN5O2, yielding a molecular weight of 518 g/mol.15 Currently, pyronaridine is commercially available only as a tetraphosphate salt containing 56.93% of the free base. The dosage of pyronaridine is expressed as its salt form, where 180 mg of pyronaridine tetraphosphate corresponds to 100 mg of pyronaridine free base. Pyronaridine tetraphosphate appears as a hydroscopic yellow powder, odourless, with a bitter taste. The salt form is sparingly soluble in water (1.46% w/v), whereas the free base is very sparingly soluble in water (0.02% w/v).16 At pH 7.4, the solubility of pyronaridine tetraphosphate was calculated at 168 µM.17 The hydrophobicity index of the pyronaridine tetraphosphate (Rm 0.872) is greater than the pyronaridine base (Rm 0.773), which confirms the higher aqueous solubility of the salt form.16
Figure 1.
Molecular structure of pyronaridine.
Pyronaridine exhibits high lipophilicity under physiological pH, which decreases with decreasing pH.18 However, a large discrepancy was seen between measured and calculated logD7.4, with values of 0.2 to 0.4 versus 4.2 to 5.3, respectively.16,19 Pyronaridine is a basic compound presenting four ionization constant values (pKa). The measured pKa values by titration were 7.08, 7.39, 9.88 and 10.30, and the calculated pKa values using ADMET Predictor were 5.2, 6.39, 7.54 and 10.1.16,19 The high pKa values suggest that pyronaridine is partly ionized at physiological pH of 7.4.20 Because pyronaridine is a lipophilic base drug, it has been regarded as a lysosomotropic agent that can gradually accumulate in cell lysosomes.17
Mechanism of action and activity
Pyronaridine is a blood schizonticide and exerts its antimalarial activity by inhibiting the formation of haemozoin pigment in the parasite digestive vacuole, similar to other quinine-type antimalarial drugs.21 An in-depth review of the mechanisms of action of pyronaridine was recently published by Bailly.22
In short, in the human host, the malaria parasites grow and multiply first in the liver cells and then enter the bloodstream where they invade erythrocytes and undergo another replication.23 In the infected erythrocytes, the parasites digest haemoglobin in their food vacuole, which leads to a release of haem.23 Free haem is toxic to the malaria parasites and is therefore subsequently converted into haematin and sequestered as haemozoin (malaria pigment) through dimerization and polymerization as a detoxification process. As the parasites develop, haemozoin releases into the blood circulation and induces inflammatory responses in the human host, and causes the clinical manifestations of the disease.24
Pyronaridine targets haematin and forms a drug-haematin complex at a stoichiometry of 1:2.25 This action inhibits the polymerization/crystallization of haematin to haemozoin and interferes with the haem detoxification process of the parasite in the digestive vacuole.26 Therefore, the level of free haem increases, which promotes peroxidative reactions and leads to parasite death.27
Besides inhibiting haemozoin formation, which is certainly responsible for its antimalarial activity, pyronaridine is known to intercalate into DNA and inhibit DNA topoisomerase 2 (Topo2) enzymes.22 A structural analogy has been noticed between pyronaridine and acridine-based drugs, such as the antiparasitic drug quinacrine and the anticancer drug amsacrine, suggesting the capacity of pyronaridine to interact with DNA.28 However, whether the interaction with DNA plays a role in the antimalarial activity of pyronaridine remains unclear, with contradictory results between experimental studies.29,30
In vitro, pyronaridine has high schizonticidal activity against field isolates and laboratory-adapted P. falciparum strains originating from various geographical regions, with the mean IC50 ranging between 0.2 and 20 nM.31,32 Compared with chloroquine, pyronaridine has an additional aniline ring that could avoid efflux-linked drug resistance.25 Additionally, the concentration of pyronaridine needed for complete haematin-induced haemolysis (10 μM) was about 1% of the concentration required for chloroquine.33 Pyronaridine retained high activity against chloroquine-sensitive and chloroquine-resistant African isolates of P. falciparum. A positive correlation was observed between the response to pyronaridine and chloroquine suggesting cross-resistance.34,35 Drug combination studies showed synergy between pyronaridine and primaquine, additive effects with 4-aminoquinolines, and weak antagonism with dihydroartemisinin, antifolates or amino alcohols.34
Pharmaceutical products available
Pyramax®
Pyramax® (pyronaridine tetraphosphate-artesunate)9 is a fixed-dose ACT indicated for the blood-stage treatment of the two dominant strains of malaria: P. falciparum and P. vivax. It was co-developed by Medicines for Malaria Venture (MMV, Geneva, Switzerland) and Shin Poong Pharmaceutical Co. Ltd (Seoul, Republic of Korea). Pyramax® is a film-coated tablet formulation (180 mg pyronaridine tetraphosphate-60 mg artesunate) for adults and children over 20 kg, and available as a granule formulation (60 mg pyronaridine tetraphosphate-20 mg artesunate) for children and infants between 5 and 20 kg. The dose should be taken orally once daily for three consecutive days based on body weight following the dosing scheme as shown in Table 1. Pyramax® was granted a positive scientific opinion from the EMA under Article 58 in 2012. In the same year, it was included in the WHO’s list of prequalified medicines, and in 2017, it was added to the WHO’s Essential Medicines Lists for both adults and children.10 Currently, Pyramax® is approved in 21 countries and included in 11 national treatment guidelines in Africa and Asia.
Table 1.
Daily dosing table for Pyramax® (pyronaridine tetraphosphate-artesunate)
| Pyramax® tablet (180 mg pyronaridine tetraphosphate-60 mg artesunate) | |||
|---|---|---|---|
| Weight range (kg) | Number of tablets | Pyronaridine dose (mg)a | Pyronaridine dose (mg/kg)a |
| 20–23 | 1 | 180 | 7.8–9.0 |
| 24–44 | 2 | 260 | 8.2–15 |
| 45–64 | 3 | 540 | 8.4–12 |
| 65–90 | 4 | 720 | 8–11.1 |
| Pyramax® granule (60 mg pyronaridine tetraphosphate-20 mg artesunate) | |||
| Weight range (kg) | Number of sachets | Pyronaridine dose (mg) | Pyronaridine dose (mg/kg) |
| 5–7 | 1 | 60 | 8.57–9.0 |
| 8–14 | 2 | 120 | 8.57–15 |
| 14–19 | 3 | 180 | 8.57–12 |
Dose is expressed as pyronaridine tetraphosphate; 180 mg pyronaridine tetraphosphate (1 tablet) corresponds to 100 mg pyronaridine base.
Malaridine
Pyronaridine tetraphosphate as monotherapy is available for the Chinese market under the trade name Malaridine.36 It is formulated as an enteric-coated tablet (100 mg pyronaridine base) for oral administration and as an injectable drip (80 mg pyronaridine base/2 mL) for intramuscular (IM) administration.37 In China, Malaridine tablets are administered with a total dosage of 1200 mg divided into four doses over a 3 day course. Two doses are given on the first day at 4–6 h intervals followed by a single dose per day on the next 2 days. Malaridine drip given by IM injection has a total dosage of 100–300 mg divided into two doses given at 4–6 h intervals.38
Analytical assays
Whole blood has been the preferred biological matrix for the clinical pharmacokinetic (PK) studies of pyronaridine due to several reasons. First of all, pyronaridine targets erythrocytic malaria parasites during the blood stages of the disease. Additionally, pyronaridine exhibits a high blood-to-plasma distribution ratio of 9.0 ± 0.83 in humans and is likely affected by the ion-trapping of basic compounds.19,20,39 These features suggest plasma concentrations may underestimate the erythrocytic target exposure of pyronaridine.
Several plasma bioanalytical assays have been documented.40–43 However, in clinical studies, pyronaridine has only been quantified in whole blood. To determine pyronaridine in whole blood, early Chinese studies used a spectrofluorometric method.44 In recent bioanalytical studies, whole blood assays applying LC-UV and LC-MS/MS methods have been developed using amodiaquine or quinine as internal standards. In these assays, the required sample volume ranged between 100 and 300 µL and the lower limit of quantitation was 1.5–30 ng/mL.45–48 In most clinical trials, the whole blood assay established by Naik et al.47 was applied to determine pyronaridine concentrations in healthy volunteers as well as malaria-infected patients. This assay employed LC-MS/MS and had a lower limit of quantification of 5.7 ng/mL.47
Pharmacokinetics
Pyronaridine has mostly been administered in the form of its tetraphosphate salt, where 180 mg of pyronaridine tetraphosphate corresponds to 100 mg of pyronaridine free base. If not mentioned otherwise, pyronaridine and its dosages in the text below refer to the tetraphosphate salt. The clinical PK of pyronaridine in adults and children are further described below under ‘Clinical PK in adults’ and ‘Clinical PK in malaria-infected children’, respectively.
Absorption
Pyronaridine presents high permeability in line with its high lipophobicity. The apparent permeability coefficient (Papp) of pyronaridine has been measured in Caco-2 assays, in which A-B Papp (10−6 cm/s) and B-A Papp (10−6 cm/s) ranged from 6.46 to 16 and 4.8 to 41, respectively.17,19
The absolute bioavailability of pyronaridine has never been assessed in humans due to the lack of an IV formulation. In rats and dogs, the absolute bioavailability of pyronaridine was 42.5% and 34.5%, respectively.49 In a study where healthy subjects were given IM pyronaridine drip and oral pyronaridine in tablet and capsule formulations, the relative bioavailability of pyronaridine tablet and capsule versus IM administration was 19% and 32%, respectively.50 The relative bioavailability between pyronaridine-artesunate granules and tablets was studied in healthy adults.49 No formulation-related differences in pyronaridine exposure were identified.49 Comparing the geometric means of the pyronaridine whole blood AUC0-∞ and the Cmax between granule and tablet formulations, the ratio was 98.8% and 98.74%, respectively.49 However, the absorption was slightly faster for the granule formulation, with a Tmax of 1 h compared with 1.5 h for the tablet formulation.49
Food effect on pyronaridine PK was studied in healthy adults given oral pyronaridine-artesunate with 720 mg pyronaridine. Compared with fasting subjects, the whole blood exposure of pyronaridine increased by 20% in subjects administered pyronaridine-artesunate with a high-fat meal.51 This food effect was, however, considered clinically not significant. Therefore, no specific recommendation regarding food intake is specified for the administration of pyronaridine-artesunate.9
Distribution
Plasma protein binding of pyronaridine was high, with the fraction unbound ranging between 4% and 8% in studies of mice, rats, rabbits and dogs.45,52,53 In humans, the fraction unbound in plasma was between 0.9% and 4.9%.17,19 It is still not fully elucidated to which plasma proteins pyronaridine mainly binds.
Although pyronaridine has a high plasma protein binding, its distribution to blood cells is not restricted. In vitro and in vivo studies indicated that pyronaridine preferentially concentrates in blood cells, resulting in a whole blood to plasma concentration ratio of 2.2–2.4 in rats, 9.1–17.8 in rabbits and 2.0–2.4 in dogs.45,52,53 In humans, the average partitioning ratio of whole blood to plasma ranged from 1.6 to 9.19,54 Therefore, a shift in the equilibrium between erythrocytes and plasma or haemolysis could markedly alter drug distribution.40 The marked uptake of pyronaridine by RBCs underlines the potential effects of variation in individual haematocrit and malaria severity.
Blood distribution of pyronaridine has been suggested to be markedly different between healthy volunteers and malaria patients.49 Basic pyronaridine is trapped within the acidic P. falciparum digestive vacuole (average pH of 5.27) due to protonation.55 Additionally, pyronaridine binds to haematin within the parasite digestive vacuole.22 These characteristics are probably the most important factors affecting pyronaridine blood distribution and efficacy in malaria patients.
Extensive distribution of pyronaridine into a wide range of tissues was observed in mice, rats and rabbits, where the highest concentration was present in the liver followed by spleen and kidneys.52,53,56 Of note, pyronaridine as a lipophilic base drug tends to accumulate in the cell lysosomes due to the pH gradient.17 Recently, a quantitative whole-body radiography study in rats confirmed that pyronaridine was extensively and rapidly distributed to various organs.57 Following a single oral dose of 720 mg [14C]-pyronaridine, radioactivity was detected from 4 h post-dose in most organs, including the liver, lung, spleen, kidney and heart.57 While in the brain, only a low level of radioactivity was detected, indicating that pyronaridine diffuses poorly through the blood–brain barrier.57 Pyronaridine is mainly concentrated in the liver and RBCs, evidenced by a higher Cmax and longer t1/2 compared with those observed in other tissues, which might be beneficial for the antimalarial activity of pyronaridine.57
Metabolism
Pyronaridine, as a Mannich base antimalarial agent, possesses an aminoquinoline moiety, typically prone to metabolism by cytochrome P450 (CYP) enzymes.58 A recombinant CYP study indicated that pyronaridine may be specifically metabolized by CYP1A2, CYP2D6 and CYP3A4 enzymes,56 raising concerns about potential drug–drug interactions (see ‘Drug–drug interactions’ below). Based on ex vivo human liver microsomes, the intrinsic CL of pyronaridine ranged between 4.1 and 8.6 μL/min/mg.17,19
The metabolism of pyronaridine could nevertheless be much more complex. In vitro, pyronaridine incubated with rat and human liver microsomes yielded 11 and 9 metabolites, respectively.17,59 In healthy adults, a mass balance study identified nine primary and four secondary pyronaridine metabolites in blood, urine and faeces. None of the identified metabolites is considered active.60 The potential metabolic pathways were N-dearylation, oxidation, demethylation, glucuronidation and cysteine conjugation, in which N-dearylation was considered the most important pathway as it accounted for most metabolites.60 In blood, urine and faeces, 31.3%, 97.7% and 36.6% of the drug-related components, respectively, were products of N-dearylation.60 Interestingly, the metabolites consistent with N-dearylation were not identified in in vitro and in vivo studies of rats nor in an in vitro study with human liver microsomes.59
Excretion
Mass balance studies in rats and dogs found a mean of 83% and 36% of total dose excreted in faeces, and 2.6% and 5.5% of total dose excreted in urine, respectively.56 The overall recovery was 93% in rats and 44% in dogs over a 14 day collection period.56 In accordance with animal studies, pyronaridine is excreted to a greater extent by the faecal route than the urinary route in humans, with a mean cumulative drug recovery of 47.8% in faeces versus 23.7% in urine until 87 days post-dose.60 More than one-third of the overall faecal recovery occurred during the first 72 h post-dose.60 Excretion of pyronaridine displayed two distinct excretion phases, with around 33% of the totally administered dose (4.6% in urine and 28.1% in faeces) excreted in the first 168 h post-dose at a relatively constant rate, followed by slower excretion until 87 days post-dose.60
Clinical PK in adults
Plasma PK in healthy and malaria-infected adults
The clinical PK of pyronaridine in plasma was described in healthy adults receiving a single dose of 400 mg pyronaridine and in Thai patients (n = 5) receiving 720 mg pyronaridine under a 3 day course (Table 2).40–42 Although the sample size was rather small in these studies, the PK profiles in plasma indicated a high variability between volunteers and patients. Despite receiving a higher dose and undergoing a longer treatment regimen, the patients demonstrated markedly lower Cmax and AUC0−∞ values compared with volunteers. These differences suggest that disease-related factors might influence the PK of pyronaridine. However, it is important to note that the plasma pyronaridine determinations might underestimate the distribution and elimination of pyronaridine, as pyronaridine tends to accumulate extensively in blood cells. Therefore, in recent clinical studies, whole blood PK profiles have been preferred for pyronaridine.
Table 2.
Pharmacokinetics of pyronaridine in plasma in healthy and malaria-infected adults
| Reference | n | Regimen | PYR dose (mg) |
C
max
(ng/mL) |
T
max
(h) |
AUC0−∞(ng/mL day) |
t
1/2
(days) |
|---|---|---|---|---|---|---|---|
| Healthy adults | |||||||
| Jayaraman et al.40 | 1 | PYR single dose | 400a | 495.8 | 0.5 | 2154 | 10.4 |
| Babalola et al.42 | 1 | PYR single dose | 400b | 76.2 | 1 | 27.62c | — |
| Malaria adults | |||||||
| Ramanathan et al.41 | 5 | PYR 3 day course | 720a | 120 ± 30 | 80 | 1225 ± 545 | 8.1 ± 2 |
All values are expressed as mean ± SD unless indicated otherwise. PYR, pyronaridine tetraphosphate.
PYR in capsule formulation.
PYR in tablet formulation.
The value represents AUC0–12h.
Whole blood PK in healthy adults
The PK of pyronaridine in whole blood has been determined in healthy volunteers receiving pyronaridine-artesunate tablets containing 360–900 mg pyronaridine following a 1 or 3 day course (Table 3).54,61–63 Pyronaridine was detectable in whole blood soon after oral administration, with mean Tmax ranging between 1.42 and 4.83 h. The elimination of pyronaridine from the systemic circulation was slow, with mean terminal t1/2 ranging between 5.03 and 16.3 days. The large variability in t1/2 was likely due to the wide sampling period across studies, ranging from 10 to 42 days. In the Phase I dose ascending study, the mean dose-corrected AUC0-∞ [AUC0-∞/dose (day/mL × 10−6)] in healthy volunteers receiving 360, 540, 720 and 900 mg of pyronaridine was 0.7, 0.6, 0.72 and 0.96, respectively.54 Therefore, dose proportionality was suggested for pyronaridine, although the drug exposure was slightly greater than dose-proportional in the highest dose group compared with the other lower dose groups.54 In healthy volunteers receiving the same dose level of pyronaridine (540 mg) for 1 and 3 days, Cmax was 1.17 to 1.43-fold higher, and AUC0-∞ was 1.8 to 3.9-fold higher for the latter.61–63 However, high variability in AUC0-∞ was observed after repeated doses, showing both large interindividual variability as well as more than 2-fold differences across studies.
Table 3.
Pharmacokinetics of pyronaridine in whole blood in healthy adults
| Reference | n | Regimen | PYR daily dose (mg) |
C
max
(ng/mL) |
T
max
(h) |
AUC0−∞ (ng/mL day) |
t
1/2
(days) |
|---|---|---|---|---|---|---|---|
| Phase I dose ascending study54,a | 7 | PA 6:2 mg/kg single dose |
360 | 186 ± 84.5 | 2.19 ± 1.63 | 258.5 ± 218.2 | 10.29 ± 10.63 |
| Phase I dose ascending study54,a | 7 | PA 9:3 mg/kg single dose |
540 | 262 ± 84.7 | 2.05 ± 1.34 | 319.5 ± 103.4 | 7.83 ± 4.54 |
| Phase I dose ascending study54,a | 7 | PA 12:4 mg/kg single dose |
720 | 467 ± 217 | 1.62 ± 0.30 | 517.9 ± 170.3 | 6.25 ± 1.51 |
| Phase I dose ascending study54,a | 7 | PA 15:5 mg/kg single dose |
900 | 792 ± 321 | 4.83 ± 4.47 | 868.4 ± 201.6 | 7.00 ± 1.99 |
| Morris et al.60,b | 6 | PA 12:4 mg/kg single dose |
720 | 266 ± 89 | 3.4 ± 3.6 | 374 ± 75 | 5.03 ± 2.19 |
| Morris et al.63,c | 16 | PA 9:3 mg/kg 3 day course |
540 | 377.2 ± 160.7 | 1.42 ± 0.36 | 1248 ± 429 | 13.1 ± 2.68 |
| Morris et al.61,d | 13 | PA 9:3 mg/kg 3 day course |
540 | 307.6 ± 110.7 | 1.5 (1–12) | 988 ± 266 | 14.5 ± 6.23 |
| Jittamala et al.62,e | 15 | PA 9:3 mg/kg 3 day course |
540 | 341 [226, 571] |
1.50 [1.00, 6.00] |
579.2 [462.5, 1000] |
16.3 [10.9, 26.5] |
All values are expressed as mean ± SD unless indicated otherwise. PA, pyronaridine-artesunate tablet; PYR, pyronaridine tetraphosphate.
Phase I dose ascending study of pyronaridine-artesunate in Korean healthy volunteers (SP-C-001-03)64 reported in PhD dissertation of Wattanavijitkul.54 The sampling period in this study was 10 days.
Mass balance study of pyronaridine in healthy volunteers with a sampling period of 42 days.
A drug–drug interaction study between pyronaridine-artesunate and ritonavir.63 Parameters were based on the first pyronaridine-artesunate dosing period of the pyronaridine-artesunate alone arm. The sampling period in this study was 42 days.
A drug–drug interaction study between pyronaridine-artesunate and metoprolol.61 Parameters were based on the pyronaridine-artesunate alone arm. The sampling period in this study was 42 days.
A drug–drug interaction study between pyronaridine-artesunate and primaquine. Parameters were based on the pyronaridine-artesunate alone group. The sampling period in this study was 42 days. Values are reported for this study as median [minimum, maximum].
Population PK analyses of pyronaridine in healthy adults have been described in several dissertations based on a pooled dataset of 258 volunteers, who received pyronaridine-artesunate tablets with pyronaridine doses of 360 to 900 mg.54,65,66 In the most recent dissertation, the PK of pyronaridine was described by a two-compartment model with first-order absorption and elimination.67 Body weight and age were included as covariates on apparent clearance (CL/F) described by power relationships. The parameter estimates of this model are shown in Table 4. The initial distribution half-life (t½α) and terminal elimination half-life (t½β) derived from the model were 0.348 ± 0.167 days and 13.74 ± 4.29 days, respectively.66
Table 4.
Population pharmacokinetic model in healthy and malaria-infected adults66
| Parameter | Parameter estimate for healthy subjects (relative SE, %) |
Additive effect of infection on parameters in malaria patients (RSE, %)a | Between-subject variability (RSE, %) |
|---|---|---|---|
| Clearance (CL/F, L/day)b | 482 (4.54) | +584 (19.3) | 0.15 (9.8) |
| Volume of central compartment (V2/F, L) | 972 (8.00) | +8060 (4.58) | 0.381 (7.85) |
| Intercompartmental clearance (Q/F, L/day) | 1350 (1.3) | — | — |
| Volume of peripheral compartment (V3/F, L) | 6560 (4.44) | +10 700 (37.6) | 0.19 (15.7) |
| Absorption rate (ka, day−1) | 20.8 (7.26) | — | 0.486 (17.3) |
| Covariate model | |||
| Power exponent of age on CL/F | 0.130 (55.9) | — | — |
| Power exponent of weight on CL/F | 0.463 (36.3) | — | — |
| Residual error model | |||
| Additive error model [ln(ng/mL)] | 0.168 (0.7) | — | — |
RSE, relative standard error.
Apparent clearance (CL/F), volume of central compartment (V2/F) and volume of peripheral compartment (V3/F) in malaria patients were 1066 L/day, 9032 L and 17 260 L, respectively.
Parameters normalized to a body weight of 58 kg and age of 26 years.
Whole blood PK in malaria-infected adults
For malaria-infected patients, the PK of pyronaridine in whole blood after oral administration was first reported in an early Chinese study from 1987 in which patients received 600 mg of Malaridine either by an enteric-coated tablet (n = 3) or a capsule (n = 3) formulation.50 For the tablet formulation mean Cmax was 130 ± 32 at a Tmax of 14.0 ± 0.3 h, and for the capsule formulation mean Cmax was 255 ± 144 ng/mL at a Tmax of 4.72 ± 0.26 h.50 In the same study, the PK of pyronaridine was also studied in patients (n = 4) given 206 mg Malaridine via IM administration. The drug was rapidly absorbed, with a mean Cmax of 525 ± 104 ng/mL at a Tmax of 0.66 ± 0.21 h, followed by a biphasic elimination with t½α of 1.0 ± 0.3 h and t½β of 63 ± 5 h.50
More recently, the whole blood PK of pyronaridine in malaria-infected patients receiving 360, 540 or 720 mg pyronaridine once daily for 3 days was reported by the manufacturer in their application for inclusion in the WHO model list for Essential Medicines (Table 5).67 The mean AUC0-∞ in patients receiving 360, 540 and 720 mg pyronaridine over a 3 day course was 749 ± 603, 1036 ± 286 and 1134 ± 624 ng/mL day, respectively.67 Compared with healthy volunteers given the same regimen (e.g. 540 mg once daily for 3 days), the AUC0-∞ was similar, whereas the Cmax after the third dose was 50%–59% lower and the Tmax was 4-fold higher in patients.
Table 5.
Pharmacokinetics of pyronaridine in whole blood in malaria-infected adults
| Reference | n | Regimen | PYR daily dose (mg) |
C
max
(ng/mL) |
T
max
(h) |
AUC0−∞ (ng/mL day) |
t
1/2
(days) |
|---|---|---|---|---|---|---|---|
| Phase II study67,a | 5 | PA 6:2 mg/kg 3 day course |
360 | 91.9 ± 30.7 | 5.3 ± 2.0 | 749 ± 603 | 19.1 ± 5.9 |
| Phase II study67,a | 5 | PA 9:3 mg/kg 3 day course |
540 | 156.8 ± 57 | 6.2 ± 6.3 | 1036 ± 286 | 15.9 ± 5.0 |
| Phase II study67,a | 6 | PA 12:4 mg/kg 3 day course |
720 | 226.1 ± 185.6 | 7.6 ± 4.9 | 1134 ± 624 | 14.6 ± 6.6 |
All values are expressed as mean ± SD unless indicated otherwise. PA, pyronaridine-artesunate tablet; PYR, pyronaridine tetraphosphate.
Phase II study (SP-C-002-05) of pyronaridine-artesunate.67
Later studies, mainly made available through published PhD dissertations, did however indicate substantial deviations in the PK between healthy subjects and malaria-infected patients, suggesting large disease-associated differences. Pyronaridine PK data from 311 malaria-infected patients who received 360, 540 or 720 mg pyronaridine were analysed in various subsequent population PK analyses all reported in PhD dissertations.54,65,66 In the latest analysis, large differences in PK parameters between volunteers and patients were characterized (Table 4). In patients, CL/F, apparent volume of central compartment (V2/F), and apparent volume of peripheral compartment (V3/F) were found to be 584 L/day (≈2.2-fold), 8060 L (≈9.3-fold) and 10 600 L (≈2.6-fold) higher than in healthy subjects, respectively. The derived t½α was 2.49 ± 1.17 days, and t½β was 28.96 ± 4.77 days in malaria-infected patients, which is 7.2 and 2.1 times greater than in healthy subjects, respectively.
In malaria-infected patients, the accumulation of pyronaridine in RBCs is likely caused by the binding of pyronaridine to haematin, resulting in a drug-haematin complex in the parasite digestive vacuole within RBCs.25 This effect is expected to lead to decreased CL/F and increased V/F in whole blood. However, the population PK analysis indicated an almost 2-fold faster CL/F and lower AUC0-∞ in malaria-infected patients, which seems counterintuitive in combination with the larger V/F and longer t1/2.66 Although no specific mechanistic theories have been proposed for this, the greater CL/F and V/F found in malaria patients could also be the result of decreased bioavailability.
Clinical PK in malaria-infected children
Children under 5 years of age are one of the most vulnerable and highly affected patient populations. In Africa alone, malaria causes around 1 million deaths in this population annually.13 Currently, pyronaridine-artesunate tablets are indicated for children above 20 kg, and pyronaridine-artesunate granules are indicated for children weighing 5–20 kg following the dosing scheme described in Table 1.8 Whole blood PK characteristics of pyronaridine in paediatrics were first investigated in a Phase II study conducted in Gabon.68 The efficacy and safety of pyronaridine-artesunate granules were subsequently studied in infants and children aged >0.5 years with body weights in the range of 5–20 kg.69,70
In the Gabonese study, children aged 2 to 14 years were administered pyronaridine-artesunate tablets or granules once daily for 3 days (Table 6).68 The mean dose-corrected AUC0-∞ [AUC0-∞/dose (day/mL × 10−6)] in children with malaria receiving pyronaridine-artesunate tablets containing 144, 216 and 288 mg of pyronaridine was 6.1, 5.0 and 6.1, respectively, suggesting dose-proportional exposure. In malaria-infected children, both Cmax and AUC0-∞ were comparable to those in malaria-infected adults who received the same dose regimen (Tables 5 and 6). The mean t1/2 in children ranged from 6.6–9 h,68 which is shorter compared with the range of 14.6–19.1 h reported in adults.67 The shorter t1/2 might indicate a more than proportional effect of weight and/or age effect on the CL and V of the drug in paediatric patients.
Table 6.
Pharmacokinetics of pyronaridine in whole blood in malaria-infected children68
| Reference | n | Regimen | PYR dose (mg) |
C
max
(ng/mL) |
T
max
(h) |
AUC0−∞ (ng/mL day) |
t
1/2
(days) |
|---|---|---|---|---|---|---|---|
| Ramharter et al.68 | 13 | PA 6:2 mg/kg tablet 3 day course |
144 | 86 ± 51 | 2.7 ± 1.9 | 734.29 ± 300.13 | 9 ± 2.5 |
| Ramharter et al.68 | 14 | PA 9:3 mg/kg tablet 3 day course |
216 | 119 ± 53 | 3.2 ± 2.4 | 907.79 ± 283.17 | 7.0 ± 1.9 |
| Ramharter et al.68 | 15 | PA 12:4 mg/kg tablet 3 day course |
288 | 339 ± 172 | 3.1 ± 1.2 | 1473.33 ± 465.13 | 6.6 ± 2.0 |
| Ramharter et al.68 | 15 | PA 9:3 mg/kg granulea 3 day course |
180 | 168 ± 57 | 2.4 ± 1.3 | 1054.8 ± 345.25 | 6.7 ± 1.8 |
All values are expressed as mean ± SD unless indicated otherwise. PA, pyronaridine-artesunate tablet; PYR, pyronaridine tetraphosphate.
Children who received PA granule had a mean weight of 17 ± 5 kg, lower than the children who received PA tablet, with mean weight of 19 ± 6 kg.68
The PK parameters of children who received pyronaridine in granule versus tablet formulations were comparable, with the exception of a 1.4-fold greater Cmax in the granule group (Table 6).68 Additionally, the tolerability, safety and efficacy of both formulations were reported to be similar in paediatric patients.68
Population PK of pyronaridine in paediatrics was characterized in a pooled analysis, including data from 349 patients with malaria younger than 16 years of age treated with pyronaridine-artesunate tablets or granules.71 The PK of pyronaridine in this population was described by a two-compartment model with first-order absorption and elimination. Allometric scaling was implemented to address the effect of body size using body weight as descriptor on clearance and volume parameters. Age was included as a covariate on V3/F with an exponent of 0.624, indicating an age-dependent increase in the peripheral volume of distribution. The final parameter estimates of this study are listed in Table 7. The absorption rate constant (ka) for the tablet formulation was 17.9 days−1, comparable to the value reported in adults.66 The ka for granule formulation was 2.63-fold higher than for the tablet formulation.71 Following the current weight-based dosing scheme (Table 1), simulated AUC0-∞ values were comparable among patients weighting 5–60 kg, with median values ranging from 1280 to 1560 ng/mL day.71
Table 7.
Population pharmacokinetic model in malaria-infected children71
| Parameter | Estimate (RSE, %) | Between-subject variability (RSE, %) |
|---|---|---|
| Clearance (CL/F, L/day)a | 377 (6.58) | 40.7% (26.7) |
| Volume of central compartment (V2/F, L)a | 2230 (6.59) | 99.6% (8.76) |
| Intercompartmental clearance (Q/F, L/day)a | 804 (11.2) | — |
| Volume of peripheral compartment (V3/F, L)a,b | 3230 (15.0) | 50.6% (48.4) |
| Absorption rate (ka, day−1) | 17.9 (11.7) | 65.8% (26.6) |
| Covariate model | ||
| Power exponent of age on V3/F | 0.624 (38.6) | — |
| Fold-change in ka for granule formulation | 2.63 (37.8) | — |
| Residual error model | ||
| Additive error model [ln(ng/mL)] | 0.195 (12.1) | — |
RSE, relative standard error.
Parameters normalized to a body weight of 20 kg following allometric scaling with fixed exponents of 0.75 for clearances and 1.0 for volumes.
Parameters normalized to an age of 7 years.
Drug–drug interactions
Given that pyronaridine is a substrate for various CYP enzymes, drug–drug interactions through these enzymes have been investigated in vitro. A strong inhibitory effect of pyronaridine on CYP2D6 was found, with an IC50 of 1.1–2.32 μM.17,61 The inhibitory effect was relatively moderate for CYP3A4 (IC50 = 42.9 μM) and mild for CYP1A2, 2C9 and 2C19 (IC50 > 50 μM).17 In a study conducted in Caco-2 cell monolayers, inhibition of P-glycoprotein (P-gp) by pyronaridine was found with an IC50 of 6.9 μM.72 Based on these preclinical results, potential drug–drug interactions of pyronaridine with inhibitors or substrates of CYP2D6 and P-gp have been studied in healthy volunteers.
A drug–drug interaction study was conducted in healthy volunteers to evaluate the interaction between pyronaridine-artesunate and metoprolol, which is a probe substrate for CYP2D6.61 The co-administration of metoprolol (100 mg) with the third dose of pyronaridine (540 or 720 mg) of a 3 day course resulted in a 47.93% increase in the Cmax of metoprolol.61 Additionally, the AUC0-∞ of metoprolol increased by 25.60%.61 Notably, these effects were observed to have a larger magnitude in individuals classified as CYP2D6 extensive metabolizers.61 Nevertheless, the effect size of this drug–drug interaction was considered clinically non-relevant, therefore co-administration of pyronaridine-artesunate with all CYP2D6 substrates is currently allowed in product label under careful clinical monitoring.9
There is a high prevalence of HIV infection in many malaria-endemic regions, therefore drug–drug interactions between pyronaridine-artesunate and ritonavir have been studied.63 Ritonavir is both an inhibitor and substrate of CYP3A4, CYP2D6 and P-gp and can induce the expression of CYP3A4 and CYP1A2. Co-administration of pyronaridine-artesunate with ritonavir did not alter the PK of pyronaridine, but increased ritonavir exposure by 3.2-fold, suggesting decreased clearance or increased absorption of ritonavir, most likely due to P-gp inhibition by pyronaridine.63
Combination treatment of pyronaridine-artesunate with primaquine was studied in Thai volunteers.62 Primaquine is an effective gametocytocide for P. falciparum infection and is the only available hypnozoitocide for P.vivax infection. Its metabolism involves CPY3A4, CPY1A2 and CPY2D6 enzymes, sharing a similar metabolic pathway as pyronaridine.73 The co-administration of a single oral dose of primaquine with pyronaridine-artesunate did not result in relevant PK changes for pyronaridine.62 However, primaquine Cmax and AUC0-∞ significantly increased by 30% and 15%, respectively, reflecting a decreased V/F and a slightly reduced elimination rate.62 This suggested a displacement of primaquine tissue-binding sites by pyronaridine in combination with inhibition of CYP2D6 and/or CYP3A4 by pyronaridine. Primaquine is widely thought to depend on CYP-mediated metabolism for its effectiveness and potential adverse effects. Nevertheless, the specific metabolic pathways responsible for primaquine activation remain poorly understood.74 Consequently, the clinical relevance of the interaction between primaquine and pyronaridine cannot be definitively determined based on these observations.62
Pregnancy
During pregnancy, because of the associated changes in the immune system and the presence of the placenta where parasites could potentially reside, pregnant women are at a higher risk of infection and the infection can have adverse effects on both mother and fetus, including maternal anaemia, fetal loss, premature delivery, intrauterine growth retardation, delivery of low birth-weight infants, and a risk factor for death.75 Preclinical studies reported the ‘no observed adverse effect level’ of pyronaridine for embryo-fetal development at 140 mg/kg/day in rats and 40 mg/kg/day in rabbits, corresponding to human equivalent doses (HEDs) of 22.68 mg/kg/day and 12.96 mg/kg/day, respectively.76,77 Both HEDs are above those currently administered to malaria patients. However, the rate of fetal resorption in humans could be dose dependent, and extrapolating these preclinical findings should be done with caution.
Little is known about the use of pyronaridine in pregnant women. In literature, at least 40 malaria-infected patients in the second and third trimesters in China have been cured with pyronaridine.56 Administration of pyronaridine-artesunate in the first trimester of pregnancy is currently outside of the product label.9 During past clinical trials, 26 pregnant women with malaria were inadvertently treated with pyronaridine-artesunate and most of them were in the first trimester.78 It was reported that 12 of these pregnant women delivered healthy babies and 4 resulted in a spontaneous abortion.78 The efficacy, safety and PK of the conventional pyronaridine-artesunate dose regimen in pregnant women with malaria have not yet been elucidated; these are currently being investigated in the multicentre PYRAPREG clinical trial conducted in Mali, Burkina Faso, Gambia, Democratic Republic of Congo and Mozambique.78
Pharmacodynamics
In vivo schizontocidal activity of pyronaridine against a broad range of Plasmodium berghei strains resulted in an ED50 (effective dose for 50% of the population) of 0.42–0.89 mg/kg and ED90 (effective dose for 90% of the population) of 0.8–30.83 mg/kg.79 By combining pyronaridine with artesunate, a significant reduction in ED90 was observed in all strains.79 The curative activity of pyronaridine at 3, 6, 9 and 12 mg/kg was studied in mice against Plasmodium chabaudi AS strain.79 A dose of 3 mg/kg pyronaridine was not effective, whereas doses equal to or above 6 mg/kg were effective during the first 28 days post-treatment.79 With 12 mg/kg of pyronaridine, no patent parasitaemia was observed throughout 56 days post-treatment, indicating that the efficacy of pyronaridine is dose-dependent.79
The pharmacodynamics (PD) of pyronaridine was further studied using a murine blood-stage malaria model.80 In mice, a single dose of pyronaridine at 2.5–25 mg/kg presented a dose-dependent killing rate at 24 and 48 h after the initial dose, whereas doses of 50–250 mg/kg exhibited dose-independent parasite reductions of 3.5 logs at 24 h, and 5–6 logs at 48 h after initial dose.80 A complete cure was achieved at a dose of 10 mg/kg, with no parasite recrudescence within 30 days. For comparing the PD between mice and humans, a pyronaridine dose of 144 mg/kg in mice was translated to 12 mg/kg in humans based on allometric adjustment.80
Treatment failure in malaria can be caused either by recrudescence of the initial parasite strain or reinfection with a new parasite strain. The relationship between treatment outcome and pyronaridine AUC0-∞, predicted whole blood concentration at Day 7 (ConcD7) as well as at Day 14 (ConcD14) has been reported in a published PhD dissertation.65 Data from 642 malaria-infected patients with 98 (15.26%) treatment failure cases were analysed. Genotyping was performed to differentiate treatment failure due to recrudescence and reinfection.65 Predicted ConcD7 was suggested to be a predictor for the hazard of occurrence of recrudescence (P value = 0.0455) and reinfection (P value = 0.026). Associated PK targets for effective treatment were an AUC0-∞ of 106 ng/mL day, ConcD7 of 31.3 ng/mL and ConcD14 of 17.6 ng/mL, albeit with relatively poor sensitivity and specificity of 60%/60%, 60/60% and 92.86%/39.52%, respectively.65
Tolerability
Pyronaridine-artesunate has demonstrated a favourable tolerability profile in Phase II/III clinical trials and a recent Cohort Event Monitoring study in Africa.81–83 However, mild-to-moderate transient increases in ALT and AST levels were reported in a small proportion of patients. According to the public assessment report of Pyramax® provided by the EMA, during the Phase II/III studies 93 patients (3.4%) had an ALT ≥ 3 × upper limit of normal (ULN), whereas 38 patients (1.4%) had an ALT ≥ 5 × ULN.49 Additionally, there were 11 patients (0.4%) who exhibited ALT ≥ 10 × ULN and met the criteria for Hy's law (ALT >3 × ULN and total bilirubin >2 × ULN).49 The peak of ALT generally occurred between Day 3 and Day 7 after the start of treatment and normalized by Day 28.49 In a subsequent safety and efficacy report based on 2815 patients enrolled in six randomized clinical trials, it was reported that seven patients (0.2%) had ALT or AST >3 × ULN plus peak total bilirubin >2 × ULN.81
The West African Network for Clinical Trials of Antimalarial Drugs (WANECAM) conducted an extensive longitudinal study in Burkina Faso, Guinea and Mali between 2011 and 2016, in which the hepatotoxicity of pyronaridine-artesunate after the first treatment and during retreatment (≥28 days after first treatment) was assessed.82 The incidence of hepatotoxicity events (ALT >5 × ULN or meeting Hy’s law) was 1.7% (11/658) of patients after first treatment and 0.9% (4/423) of patients during the retreatment.82 None of these hepatotoxicity events required intervention or resulted in long-term consequences.82 Additional analysis of data from Burkina Faso between 2012 and 2015 indicated that retreated patients were 68% less likely to have elevated ALT.84
In a recent non-comparative Cohort Event Monitoring study of Pyramax®, the hepatic safety and tolerability of pyronaridine-artesunate were assessed in real-world conditions in Africa.83 It was found that 1.9% (158/8560) of malaria episodes occurred in patients with ALT or AST >2 × ULN, whereas no protocol-defined hepatotoxicity events were reported.83 In the updated WHO Guidelines for malaria after 2022, the hepatotoxicity concerns of pyronaridine-artesunate were no longer considered justified.13
Possible correlations between the PK properties of pyronaridine and hepatic enzyme elevations were observed in a drug–drug interaction study.63 Of a total of 34 subjects receiving pyronaridine-artesunate or a combination of pyronaridine-artesunate and ritonavir, 5 individuals showed increased ALT >3 × ULN or ALT >5 × ULN.63 These five subjects had the highest pyronaridine Cmax and AUC till the end of the dosing interval (AUC0-tau) within the studied cohort, underlining a potential impact of pyronaridine exposure on the level of hepatic enzymes. Nevertheless, all of these subjects were also co-administered ritonavir, which is known to cause hepatic enzyme elevations.63 Therefore, it remains unclear whether pyronaridine alone was responsible for the observed hepatic enzyme elevations. Additionally, pyronaridine might increase the exposure to ritonavir, which may also lead indirectly to hepatic enzyme elevation.
Post-treatment haemolytic anaemia is a known complication for various severe malaria treatments; therefore, caution should be taken for malaria patients with a low baseline haemoglobin.85 Like for other antimalarials, treatment with pyronaridine-artesunate can cause a decrease in haemoglobin of up to 2 g/dL, which usually reaches a nadir by Day 3 and recovers by Day 28.51 Because pyronaridine extensively concentrates in RBCs, variations in haemoglobin could very well affect the disposition of pyronaridine. There is limited information regarding the occurrence of post-treatment anaemia when pyronaridine is used in combination with other antimalarials that have the potential to cause haemolysis. However, in healthy subjects receiving a combination of pyronaridine-artesunate and primaquine, no significant alteration in methaemoglobin levels was found.62
Conclusion
Pyronaridine has been used as an antimalarial drug against P. falciparum and P. vivax since the 1970s. However, before the 1990s, its application and clinical studies were restricted to China. Interest in pyronaridine-artesunate increased due to rising MDR P. falciparum. In 2012, pyronaridine-artesunate was granted a positive scientific opinion under the EMA Article 58 procedure. In 2017, pyronaridine-artesunate was added to the WHO Model List of Essential Medicines and Model List of Essential Medicines for Children, respectively. In 2022, the renewed WHO guidelines for malaria strongly recommended pyronaridine-artesunate as a safe and efficacious ACT for the treatment of uncomplicated malaria in adults and children.
In recent years, various clinical trials have been conducted to assess the efficacy and safety of pyronaridine-artesunate in both adults and children. However, our understanding of pyronaridine PK is still relatively limited. It is known that pyronaridine highly concentrates in the blood cells of healthy adults, which makes whole blood a preferable matrix for studying clinical PK. Based on PK profiles evaluated in whole blood, pyronaridine is rapidly absorbed with the peak concentration observed around 2 h after oral administration. After absorption, pyronaridine extensively distributes to blood and tissues, with a terminal t1/2 around 13–15 days. In general, the peak concentration and the exposure to pyronaridine follow a dose-proportional pattern. Potential drug–drug interactions of pyronaridine have been underlined, where caution is advised when co-administering pyronaridine with substrates of CYP2D6 and P-gp. Pyronaridine was found to increase the exposure of ritonavir, metoprolol and primaquine, likely due to the inhibition of CYP2D6 and P-gp regulated efflux.
Comparing the PK properties of pyronaridine between healthy volunteers and malaria-infected patients, Cmax and AUC0-∞ were lower in malaria patients, suggesting a slower pyronaridine absorption rate and poor bioavailability in malaria patients. In addition, malaria patients had a significantly larger V/F and longer t1/2, which might reflect the complexation of the drug to the haematin that is produced when parasites digest haemoglobin. Although it is challenging to make direct comparisons between the various highly heterogeneous studies and draw robust conclusions, the different PK profiles in healthy and malaria-infected subjects might underline the importance of disease-related effects possibly affecting bioavailability, distribution and clearance of the compound, such as variations in parasite growth, haematocrit and haemolysis. Variations in demographics might also (partially) account for the high interindividual variability in PK parameters found in both healthy and malaria-infected populations. Nevertheless, population PK studies comparing healthy subjects and malaria-infected patients are lacking or remained unpublished as non-peer-reviewed theses, and the possible factors that might account for the observed variation between populations and within individuals remain to be investigated.
Recent studies of pyronaridine-artesunate focus on its application in special populations such as children and pregnant women, as they are more vulnerable to malaria infection and make up a large part of the global burden of malaria. Based on the current weight-based dosing scheme for pyronaridine-artesunate, children ≥20 kg and <20 kg are administered the drug in tablet and granule formulation, respectively. Only one peer-reviewed published study reported the PK of pyronaridine in children. Potential body size effect (allometric scaling using body weight as descriptor) on CL/F and V/F as well as an additional age effect on V/F were reported. Although the granule formulation exhibited a more than 2-fold higher absorption rate compared with the tablet formulation, the overall drug exposures were comparable among children weighing from 5 to 60 kg. Pyronaridine-artesunate in either tablet or granule formulation is not approved for use in pregnancy. A clinical study is currently ongoing to understand the efficacy, safety and PK of pyronaridine in pregnant women with malaria.
In conclusion, although pyronaridine has been used for decades, the clinical PK of pyronaridine in different populations remain unclear. Moreover, no clear PK target has been associated with an effective PD response. Further in-depth population PK-PD studies to investigate the potential impacts of disease-, age- and population-related physiological changes on the PK of pyronaridine and to define PK-PD relationships are required.
Contributor Information
Wan-Yu Chu, Department of Pharmacy and Pharmacology, Netherlands Cancer Institute, Amsterdam, The Netherlands.
Thomas P C Dorlo, Department of Pharmacy and Pharmacology, Netherlands Cancer Institute, Amsterdam, The Netherlands; Department of Pharmacy, Uppsala University, Uppsala, Sweden.
Funding
W.C. and T.P.C.D. were supported through the PYRAPREG consortium, which is part of the EDCTP2 programme supported by the European Union (RIA2017MC-2025). T.P.C.D. acknowledges funding from the Dutch Research Council (Veni grant no. 91617140) and the Swedish Research Council (grant no. 2022-01251).
Transparency declarations
None to declare. W.C. and T.P.C.D. have no conflicts of interest.
References
- 1. Zheng XY, Chen C, Gao FHet al. . Synthesis of new antimalarial drug pyronaridine and its analogues. Acta Pharm Sin 1982; 17: 118–25. [PubMed] [Google Scholar]
- 2. Zheng XY, Xia Y, Gao FHet al. . Synthesis of 7351, a new antimalarial drug [author’s translation]. Acta Pharm Sin 1979; 14: 736–7. [PubMed] [Google Scholar]
- 3. Trape JF, Pison G, Preziosi MPet al. . Impact of chloroquine resistance on malaria mortality. C R Acad Sci III 1998; 321: 689–97. 10.1016/s0764-4469(98)80009-7 [DOI] [PubMed] [Google Scholar]
- 4. Winstanley P. Pyronaridine: a promising drug for Africa? Lancet 1996; 347: 2–3. 10.1016/S0140-6736(96)91548-2 [DOI] [PubMed] [Google Scholar]
- 5. Ringwald P, Bickii J, Basco L. Randomised trial of pyronaridine versus chloroquine for acute uncomplicated falciparum malaria in Africa. Lancet 1996; 347: 24–8. 10.1016/S0140-6736(96)91558-5 [DOI] [PubMed] [Google Scholar]
- 6. Ringwald P, Bickii J, Basco LK. Efficacy of oral pyronaridine for the treatment of acute uncomplicated falciparum malaria in African children. Clin Infect Dis 1998; 26: 946–53. 10.1086/513942 [DOI] [PubMed] [Google Scholar]
- 7. Balint GA. Artemisinin and its derivatives: an important new class of antimalarial agents. Pharmacol Ther 2001; 90: 261–5. 10.1016/S0163-7258(01)00140-1 [DOI] [PubMed] [Google Scholar]
- 8. MMV (Medicinces for Malaria Venture) . Pyramax® (pyronaridine-artesunate) for the treatment of acute uncomplicated malaria in adults and children. https://www.mmv.org/mmv-pipeline-antimalarial-drugs/pyronaridine-artesunate
- 9. European Medicines Agency (EMA) . Pyramax: opinion on medicine for use outside EU. https://www.ema.europa.eu/en/opinion-medicine-use-outside-EU/human/pyramax
- 10. European Medicines Agency (EMA) . Electronic Essential Medicines List (eEML)-artesunate + pyronaridine tetraphosphate. https://list.essentialmeds.org/medicines/377
- 11. World Health Organization . WHO guidelines for the treatment of malaria, 3rd ed. WHO, 2015. https://apps.who.int/iris/handle/10665/162441 [PubMed] [Google Scholar]
- 12. World Health Organization . The use of artesunate-pyronaridine for the treatment of uncomplicated malaria. WHO. https://apps.who.int/iris/handle/10665/328762 [Google Scholar]
- 13. World Health Organization . WHO guidelines for malaria. WHO, 2023.https://www.who.int/publications/i/item/guidelines-for-malaria
- 14. Valdés AF-C. Acridine and acridinones: old and new structures with antimalarial activity. Open Med Chem J 2011; 5: 11–20. 10.2174/1874104501105010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Center for Biotechnology Information . PubChem compound summary for CID 25245913. https://pubchem.ncbi.nlm.nih.gov/compound/25245913
- 16. Adegoke OA, Babalola CP, Oshitade OSet al. . Determination of the physicochemical properties of pyronaridine—a new antimalarial drug. Pak J Pharm Sci 2006; 19: 1–6. [PubMed] [Google Scholar]
- 17. Lane TR, Massey C, Comer JEet al. . Repurposing the antimalarial pyronaridine tetraphosphate to protect against ebola virus infection. PLoS Negl Trop Dis 2019; 13: e0007890. 10.1371/journal.pntd.0007890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruscoe JE, Tingle MD, O’Neill PMet al. . Effect of disposition of Mannich antimalarial agents on their pharmacology and toxicology. Antimicrob Agents Chemother 1998; 42: 2410–6. 10.1128/AAC.42.9.2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Charman SA, Andreu A, Barker Het al. . An in vitro toolbox to accelerate anti-malarial drug discovery and development. Malar J 2020; 19: 1–27. 10.1186/s12936-019-3075-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siebert GA, Hung DY, Chang Pet al. . Ion-trapping, microsomal binding, and unbound drug distribution in the hepatic retention of basic drugs. J Pharmacol Exp Ther 2004; 308: 228–35. 10.1124/jpet.103.056770 [DOI] [PubMed] [Google Scholar]
- 21. Kumar S, Guha M, Choubey Vet al. . Antimalarial drugs inhibiting hemozoin (β-hematin) formation: a mechanistic update. Life Sci 2007; 80: 813–28. 10.1016/j.lfs.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 22. Bailly C. Pyronaridine: an update of its pharmacological activities and mechanisms of action. Biopolymers 2021; 112: e23398. 10.1002/bip.23398 [DOI] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention (CDC) . Malaria. https://www.cdc.gov/parasites/malaria/index.html
- 24. Olivier M, Van Den Ham K, Shio MTet al. . Malarial pigment hemozoin and the innate inflammatory response. Front Immunol 2014; 5: 25. 10.3389/fimmu.2014.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Auparakkitanon S, Chapoomram S, Kuaha Ket al. . Targeting of hematin by the antimalarial pyronaridine. Antimicrob Agents Chemother 2006; 50: 2197–200. 10.1128/AAC.00119-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Auparakkitanon S, Noonpakdee W, Ralph RKet al. . Antimalarial 9-anilinoacridine compounds directed at hematin. Antimicrob Agents Chemother 2003; 47: 3708–12. 10.1128/AAC.47.12.3708-3712.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Combrinck JM, Mabotha TE, Ncokazi KKet al. . Insights into the role of heme in the mechanism of action of antimalarials. ACS Chem Biol 2013; 8: 133–7. 10.1021/cb300454t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bourdouxhe-Housiaux C, Colson P, Houssier Cet al. . Interaction of a DNA-threading netropsin-amsacrine combilexin with DNA and chromatin. Biochemistry 1996; 35: 4251–64. 10.1021/bi9528098 [DOI] [PubMed] [Google Scholar]
- 29. Chavalitshewinkoon P, Wilairat P, Gamage Set al. . Structure-activity relationships and modes of action of 9-anilinoacridines against chloroquine-resistant Plasmodium falciparum in vitro. Antimicrob Agents Chemother 1993; 37: 403–6. 10.1128/AAC.37.3.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Auparakkitanon S, Wilairat P. Cleavage of DNA induced by 9-anilinoacridine inhibitors of topoisomerase II in the malaria parasite Plasmodium falciparum. Biochem Biophys Res Commun 2000; 269: 406–9. 10.1006/bbrc.2000.2305 [DOI] [PubMed] [Google Scholar]
- 31. Kemirembe K, Cabrera M, Cui L. Interactions between tafenoquine and artemisinin-combination therapy partner drug in asexual and sexual stage Plasmodium falciparum. Int J Parasitol Drugs Drug Resist 2017; 7: 131–7. 10.1016/j.ijpddr.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rueangweerayut R, Phyo AP, Uthaisin Cet al. . Pyronaridine–artesunate versus mefloquine plus artesunate for malaria. N Engl J Med 2012; 366: 1298–309. 10.1056/NEJMoa1007125 [DOI] [PubMed] [Google Scholar]
- 33. Auparakkitanon S, Wilairat P. Antimalarial activity of concanamycin A alone and in combination with pyronaridine. Southeast Asian J Trop Med Public Health 2006; 37: 619–21. [PubMed] [Google Scholar]
- 34. Ringwald P, Eboumbou ECM, Bickii Jet al. . In vitro activities of pyronaridine, alone and in combination with other antimalarial drugs, against Plasmodium falciparum. Antimicrob Agents Chemother 1999; 43: 1525–7. 10.1128/AAC.43.6.1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pradines B, Tall A, Parzy D, et al. . In-vitro activity of pyronaridine and amodiaquine against African isolates (Senegal) of Plasmodium falciparum in comparison with standard antimalarial agents. J Antimicrob Chemother 1998; 42: 333–9. 10.1093/jac/42.3.333 [DOI] [PubMed] [Google Scholar]
- 36. Zhu H, Wang Y, Hussain A, et al. . Nanodiamond mediated co-delivery of doxorubicin and malaridine to maximize synergistic anti-tumor effects on multi-drug resistant MCF-7/ADR cells. J Mater Chem B 2017; 5: 3531–40. 10.1039/C7TB00449D [DOI] [PubMed] [Google Scholar]
- 37. Chang C, Lin-Hua T, Jantanavivat C. Studies on a new antimalarial compound: Pyronaridine. Trans R Soc Trop Med Hyg 1992; 86: 7–10. 10.1016/0035-9203(92)90414-8 [DOI] [PubMed] [Google Scholar]
- 38. Chen C. Development of antimalarial drugs and their application in China: a historical review. Infect Dis Poverty 2014; 3: 9. 10.1186/2049-9957-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. White RE. Role of ADME/PK in drug discovery, safety assessment, and clinical development. Compr Med Chem III 2017; 4–8: 1–33. 10.1016/B978-0-12-409547-2.12364-9 [DOI] [Google Scholar]
- 40. Jayaraman SD, Ismail S, Nair NKet al. . Determination of pyronaridine in blood plasma by high-performance liquid chromatography for application in clinical pharmacological studies. J Chromatogr B Biomed Appl 1997; 690: 253–7. 10.1016/S0378-4347(96)00410-0 [DOI] [PubMed] [Google Scholar]
- 41. Ramanathan S, Karupiah S, Nair NKet al. . A new and simple solid-phase extraction method for LC determination of pyronaridine in human plasma. J Chromatogr B Anal Technol Biomed Life Sci 2005; 824: 45–50. 10.1016/j.jchromb.2005.06.034 [DOI] [PubMed] [Google Scholar]
- 42. Babalola CP, Scriba GKE, Sowunmi Aet al. . Liquid chromatographic determination of pyronaridine in human plasma and oral dosage form. J Chromatogr B Anal Technol Biomed Life Sci 2003; 795: 265–72. 10.1016/S1570-0232(03)00591-9 [DOI] [PubMed] [Google Scholar]
- 43. Hodel EM, Zanolari B, Mercier Tet al. . A single LC-tandem mass spectrometry method for the simultaneous determination of 14 antimalarial drugs and their metabolites in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877: 867–86. 10.1016/j.jchromb.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 44. Feng Z, Wang CY. [Spectrofluorometric determination of pyronaridine, an antimalarial]. Zhongguo Yao Li Xue Bao 1986; 7: 354–7. [PubMed] [Google Scholar]
- 45. Chen YC, Fleckenstein L. Improved assay method for the determination of pyronaridine in plasma and whole blood by high-performance liquid chromatography for application to clinical pharmacokinetic studies. J Chromatogr B Biomed Sci Appl 2001; 752: 39–46. 10.1016/S0378-4347(00)00512-0 [DOI] [PubMed] [Google Scholar]
- 46. Blessborn D, Lindegårdh N, Ericsson Öet al. . Determination of pyronaridine in whole blood by automated solid phase extraction and high-performance liquid chromatography. Ther Drug Monit 2003; 25: 264–70. 10.1097/00007691-200306000-00003 [DOI] [PubMed] [Google Scholar]
- 47. Naik H, Imming P, Schmidt MSet al. . Development and validation of a liquid chromatography-mass spectrometry assay for the determination of pyronaridine in human blood for application to clinical pharmacokinetic studies. J Pharm Biomed Anal 2007; 45: 112–9. 10.1016/j.jpba.2007.06.018 [DOI] [PubMed] [Google Scholar]
- 48. Blessborn D, Kaewkhao K, Song Let al. . Quantification of the antimalarial drug pyronaridine in whole blood using LC–MS/MS—increased sensitivity resulting from reduced non-specific binding. J Pharm Biomed Anal 2017; 146: 214–9. 10.1016/j.jpba.2017.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. European Medicines Agency (EMA) . Medicinal products for human use (CHMP). Pyramax: public assessment report. 2012. https://www.ema.europa.eu/en/documents/outside-eu-assessment-report/pyramax-public-assessment-report_en.pdf
- 50. Feng Z, Wu ZF, Wang CYet al. . Pharmacokinetics of pyronaridine in malaria patients. Zhongguo Yao Li Xue Bao 1987; 8: 543–6. [PubMed] [Google Scholar]
- 51. European Medicines Agency (EMA) . Medicinal products for human use (CHMP). Pyramax: summary of product characteristics. 2012. https://www.ema.europa.eu/en/documents/outside-eu-product-information/pyramax-product-information_en.pdf
- 52. Feng Z, Wu ZF, Wang CYet al. . Distribution and excretion of 3H-pyronaridine in mice. Acta Pharm Sin 1988; 23: 629–32. [PubMed] [Google Scholar]
- 53. Feng Z, Jiang NX, Wang CYet al. . [Pharmacokinetics of pyronaridine, an antimalarial in rabbits]. Yao Xue Xue Bao 1986; 21: 801–5. [PubMed] [Google Scholar]
- 54. Wattanavijitkul T. Population pharmacokinetics of pyronaridine in the treatment of malaria. PhD thesis. The University of Iowa, 2010. https://iro.uiowa.edu/esploro/outputs/doctoral/Population-pharmacokinetics-of-pyronaridine-in-the/9983777093402771
- 55. Bakar NA. Measuring pH of the Plasmodium falciparum digestive vacuole by flow cytometry. Trop Biomed 2015; 32: 485–93. [PubMed] [Google Scholar]
- 56. Croft SL, Duparc S, Arbe-Barnes SJet al. . Review of pyronaridine anti-malarial properties and product characteristics. Malar J 2012; 11: 270. 10.1186/1475-2875-11-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Park SH, Pradeep K. Absorption, distribution, excretion, and pharmacokinetics of C14-pyronaridine tetraphosphate in male and female sprague-dawley rats. J Biomed Biotechnol 2010; 2010: 590707. 10.1155/2010/590707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. O’Neill PM, Mukhtar A, Stocks PAet al. . Isoquine and related amodiaquine analogues: a new generation of improved 4-aminoquinoline antimalarials. J Med Chem 2003; 46: 4933–45. 10.1021/jm030796n [DOI] [PubMed] [Google Scholar]
- 59. Lee J, Son J, Chung SJet al. . In vitro and in vivo metabolism of pyronaridine characterized by low-energy collision-induced dissociation mass spectrometry with electrospray ionization. J Mass Spectrom 2004; 39: 1036–43. 10.1002/jms.663 [DOI] [PubMed] [Google Scholar]
- 60. Morris CA, Dueker SR, Lohstroh PNet al. . Mass balance and metabolism of the antimalarial pyronaridine in healthy volunteers. Eur J Drug Metab Pharmacokinet 2015; 40: 75–86. 10.1007/s13318-014-0182-0 [DOI] [PubMed] [Google Scholar]
- 61. Morris CA, Pokorny R, Lopez-Lazaro Let al. . Pharmacokinetic interaction between pyronaridine-artesunate and metoprolol. Antimicrob Agents Chemother 2014; 58: 5900–8. 10.1128/AAC.02716-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jittamala P, Pukrittayakamee S, Ashley EAet al. . Pharmacokinetic interactions between primaquine and pyronaridine-artesunate in healthy adult Thai subjects. Antimicrob Agents Chemother 2015; 59: 505–13. 10.1128/AAC.03829-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Morris CA, Lopez-Lazaro L, Jung Det al. . Drug-drug interaction analysis of pyronaridine/artesunate and ritonavir in healthy volunteers. Am J Trop Med Hyg 2012; 86: 489–95. 10.4269/ajtmh.2012.11-0558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tan B, Naik H, Jang IJet al. . Population pharmacokinetics of artesunate and dihydroartemisinin following single-and multiple-dosing of oral artesunate in healthy subjects. Malar J 2009; 8: 304. 10.1186/1475-2875-8-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Methaneethorn J. Population pharmacokinetics and pharmacodynamics of pyronaridine. PhD thesis. University of Iowa, 2013.
- 66. Ayyoub AS. Pharmacokinetics of pyronaridine in adult and pediatric populations. PhD thesis. University of Iowa, 2016.
- 67. Shin Poong Pharmaceuticals Co. Ltd . Application for inclusion in WHO model list of essential medicine.2016.
- 68. Ramharter M, Kurth F, Schreier ACet al. . Fixed-dose pyronaridine-artesunate combination for treatment of uncomplicated falciparum malaria in pediatric patients in Gabon. J Infect Dis 2008; 198: 911–9. 10.1086/591096 [DOI] [PubMed] [Google Scholar]
- 69. Kayentao K, Doumbo OK, Pénali LKet al. . Pyronaridine-artesunate granules versus artemether-lumefantrine crushed tablets in children with Plasmodium falciparum malaria: a randomized controlled trial. Malar J 2012; 11: 364. 10.1186/1475-2875-11-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sagara I, Beavogui AH, Zongo Iet al. . Safety and efficacy of re-treatments with pyronaridine-artesunate in African patients with malaria: a substudy of the WANECAM randomised trial. Lancet Infect Dis 2016; 16: 189–98. 10.1016/S1473-3099(15)00318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ayyoub A, Methaneethorn J, Ramharter Met al. . Population pharmacokinetics of pyronaridine in pediatric malaria patients. Antimicrob Agents Chemother 2016; 60: 1450–8. 10.1128/AAC.02004-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Oga EF, Sekine S, Shitara Yet al. . Potential P-glycoprotein-mediated drug-drug interactions of antimalarial agents in Caco-2 cells. Am J Trop Med Hyg 2012; 87: 64–9. 10.4269/ajtmh.2012.11-0817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ariffin NM, Islahudin F, Kumolosasi Eet al. . Effects of MAO-A and CYP450 on primaquine metabolism in healthy volunteers. Parasitol Res 2019; 118: 1011–8. 10.1007/s00436-019-06210-3 [DOI] [PubMed] [Google Scholar]
- 74. Pybus BS, Marcsisin SR, Jin X, et al. . The metabolism of primaquine to its active metabolite is dependent on CYP 2D6. Malar J 2013; 12: 1–7. 10.1186/1475-2875-12-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Desai M, ter Kuile FO, Nosten F, et al. . Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7: 93–104. 10.1016/S1473-3099(07)70021-X [DOI] [PubMed] [Google Scholar]
- 76. Ni YC, Zhan CQ, Ha SHet al. . [The embryotoxicity of a new antimalarial pyronaridine in rats]. Yao Xue Xue Bao 1982; 17: 401–6. [PubMed] [Google Scholar]
- 77. Nair A, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016; 7: 27. 10.4103/0976-0105.177703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Anon . PYRAPREG Project homepage. https://www.pyrapreg.org/
- 79. Vivas L, Rattray L, Stewart Let al. . Anti-malarial efficacy of pyronaridine and artesunate in combination in vitro and in vivo. Acta Trop 2008; 105: 222–8. 10.1016/j.actatropica.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 80. Okoth WA, Dukes EJ, Sullivan DJ. Superior pyronaridine single-dose pharmacodynamics compared to artesunate, chloroquine, and amodiaquine in a murine malaria luciferase model. Antimicrob Agents Chemother 2018; 62: e00394-18. 10.1128/AAC.00394-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Duparc S, Borghini-Fuhrer I, Craft CJet al. . Safety and efficacy of pyronaridine-artesunate in uncomplicated acute malaria: an integrated analysis of individual patient data from six randomized clinical trials. Malar J 2013; 12: 70. 10.1186/1475-2875-12-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sagara I, Beavogui AH, Zongo Iet al. . Pyronaridine-artesunate or dihydroartemisinin-piperaquine versus current first-line therapies for repeated treatment of uncomplicated malaria: a randomised, multicentre, open-label, longitudinal, controlled, phase 3b/4 trial. Lancet 2018; 391: 1378–90. 10.1016/S0140-6736(18)30291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tona Lutete G, Mombo-Ngoma G, Assi SBet al. . Pyronaridine-artesunate real-world safety, tolerability, and effectiveness in malaria patients in 5 African countries: a single-arm, open-label, cohort event monitoring study. PLoS Med 2021; 18: e1003669. 10.1371/journal.pmed.1003669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Compaoré YD, Zongo I, Somé AFet al. . Hepatic safety of repeated treatment with pyronaridine-artesunate versus artemether-lumefantrine in patients with uncomplicated malaria: a secondary analysis of the WANECAM 1 data from Bobo-Dioulasso, Burkina Faso. Malar J 2021; 20: 64. 10.1186/s12936-021-03593-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sagara I, Piarroux R, Djimde Aet al. . Delayed anemia assessment in patients treated with oral artemisinin derivatives for uncomplicated malaria: a pooled analysis of clinical trials data from Mali. Malar J 2014; 13: 358. 10.1186/1475-2875-13-358 [DOI] [PMC free article] [PubMed] [Google Scholar]