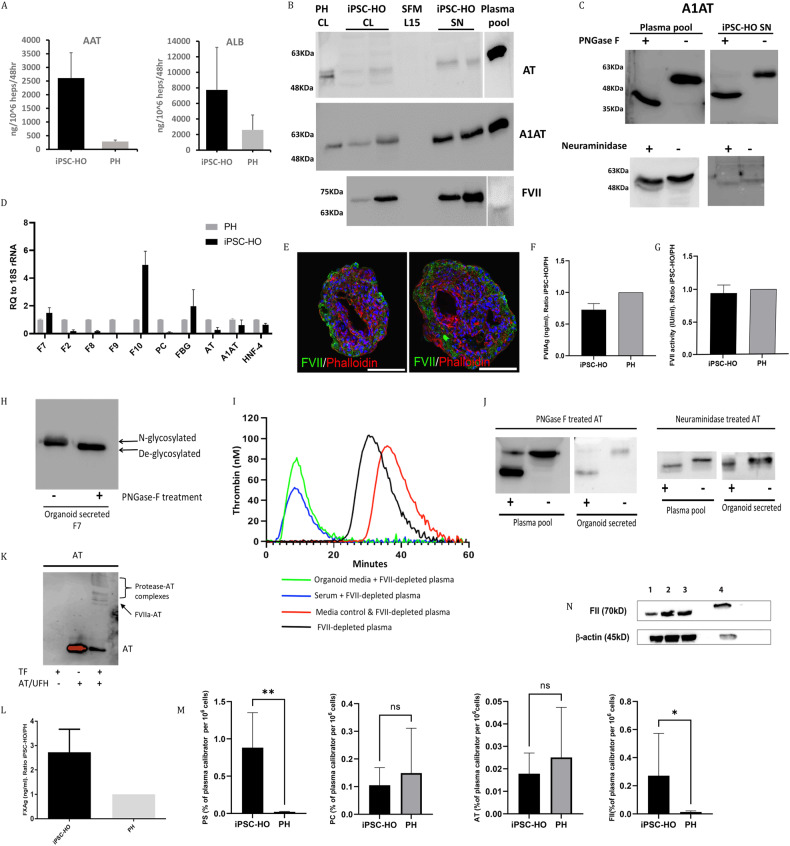
Fig. 8. Assessment of liver organoid coagulation machinery.
A Demonstration of the production and secretion of A1AT and albumin into the culture medium by D21-24 AG27-derived organoids and primary human hepatocytes over 48 hours as measured by ELISAs (n = 3, mean ± SD). B Antithrombin (AT), alpha-1-antitrypsin (A1AT) and factor VII (FVII) are present in cell lysates (Lanes 2 and 3) and supernatants (Lanes 5 and 6) derived from liver organoids assessed by SDS‒PAGE under reducing conditions. Plasma pool (Lane 7) and primary hepatocytes (Lane 1) were used as a reference; serum-free medium (SFM) L15 medium was used as a negative control (Lane 4). C Assessment of the N-glycosylation content of A1AT from the supernatant of organoids by PNGase F compared to the plasma pool. D Levels of coagulation factors and inhibitors in iPSC-derived organoids (black bars) and primary human hepatocytes (gray bars). mRNA levels of coagulation factors II, VII, VIII, IX, and X, fibrinogen (F2, F7, F8, F9, F10, FBG), the coagulation inhibitors protein C and antithrombin (PC, AT) and the hepatic markers alpha-1 antitrypsin and hepatocyte nuclear factor 4 alpha (A1AT, HNF4α) were determined using quantitative RT‒qPCR with 18 S as an endogenous control. The results are presented as the mean of the fold change expression of the respective gene. The results from three independent experiments are presented as the mean ± SD. E Immunostaining of two 50 μm cryosections showing localization of FVII (green) to the outer hepatocyte layer of the AG27-derived organoids, co-stained with phalloidin (red). Nuclei in blue, scale bars are 100 µm. F Demonstrating the production and secretion of FVII protein in the culture medium of AG27-derived liver organoids (iPSC-HO) and primary human hepatocytes (PH) as determined using ELISAs. The total concentration of FVII was adjusted to 1×106 cells, and the results are expressed as the iPSC-HO/PH ratio. The results from three independent experiments are presented as the mean ± SEM. G Demonstrating FVII activity (IU/ml), culture medium from AG27-derived iPSC-HO and PH was determined using an FVII chromogenic assay. The results were adjusted to 1×106 cells and are expressed as the iPSC-HO/PH ratio. The results from three independent experiments are presented as the mean ± SEM. H Assessment of the N-glycosylation content of secreted FVII of organoids by PNGase F treatment. I Assessment of thrombin generation in FVII-depleted plasma (black) with either serum-free medium (SFM) (red), organoid supernatant (green) or fetal bovine serum (FBS)-supplemented serum-free medium (SFM) L15 (blue). J Assessment of the N-glycosylation content of secreted AT from the supernatant of organoids by PNGase F and neuraminidase treatment compared to the human plasma pool. K Western blot analysis of AT after activation of organoid-derived supernatants by tissue factor (TF) and CaCl2 and incubation with AT and unfractionated heparin. Activated FVII-AT (FVIIa-AT) complexes are indicated by an arrow and bracket. l Left panel: FX protein (Ag) levels (ng/ml) in culture medium from iPSC-HO and PH as measured by ELISAs. The total concentration of FX was adjusted to 1×106 cells, and the results are expressed as the iPSC-HO/PH ratio. The results from three independent experiments are presented as the mean ± SEM. M Demonstrating the production and secretion of PS, PC, AT and FII proteins in the culture medium of AG27-derived liver organoids (iPSC-HO) and primary human hepatocytes (PH) as determined using the Procarta-plex assay. The total concentration of AT was adjusted to 1×106 cells, and the results are expressed as the percentage of the plasma calibrator control. The results from three independent experiments are presented as the mean ± SD. Statistical significance was assessed with the Mann‒Whitney test (*p < 0.05, **p < 0.01). N Intracellular levels of prothrombin (FII) shown by western blots, lysates from iPSC-HO (Lanes 1-3) and PH (Lane 4). Equal amounts of proteins were separated by SDS‒PAGE under reducing conditions. β-Actin was used as a loading control. The results of three independent experiments are presented.