Table 2.
Protein arginine methyltransferase 5 inhibitors.
| Drug Name | Structure | Clinical trial identifier (if applicable) |
Clinical trial stage (if applicable) |
Cancer type in trial (if applicable) |
Citation |
|---|---|---|---|---|---|
| EPZ015666/ GSK3326595 |
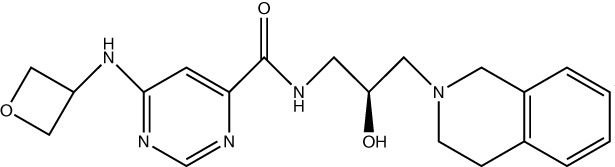
|
NCT04676516 | Phase 2 | Early-stage breast cancer | (83) |
| LLY-283 |
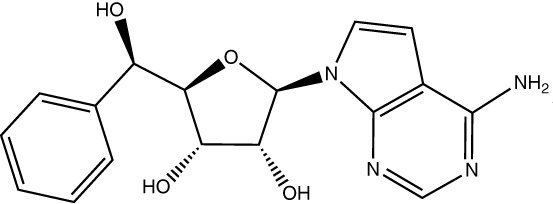
|
(85) | |||
| JNJ-64619178 |
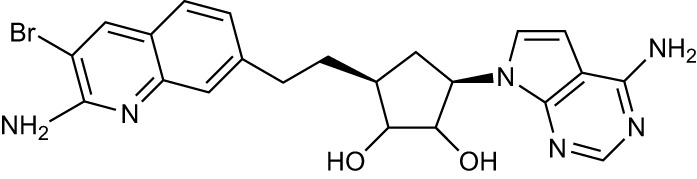
|
NCT03573310 | Phase 1 | Neoplasms, Solid tumour (adult), Non-Hodgkin Lymphoma, Myelodysplastic Syndromes | (86, 87) |
| BRD0639 |
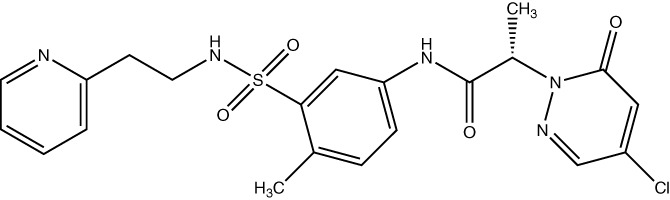
|
(90) | |||
| AMG193 | Not published | NCT05094336 | Phase 1/2 | MTAP-null solid tumours | No publications |
| TNG908 | Not published | NCT05275478 | Phase 1/2 | MTAP-null solid tumours | No publications |
| SCR-6920 | Not published | NCT05528055 | Phase 1 | Solid tumour, Non-Hodgkin Lymphoma | No publications |
| PRT543 | Not published | NCT03886831 | Phase 1 | Solid tumours/ lymphomas, Haematological malignancies |
No publications |
| PRT811 | Not published | NCT04089449 | Phase 1 | Advanced solid tumour, Recurrent Glioma | No publications |
| MRTX1719 |
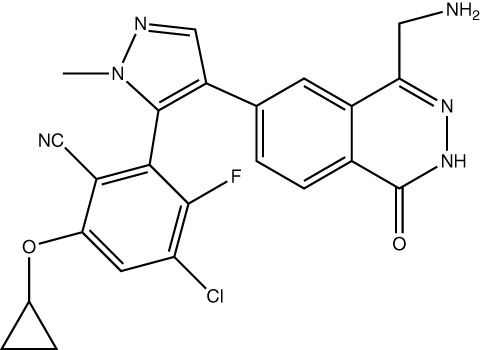
|
NCT05245500 | Phase 1/2 | Mesothelioma, Non-small cell lung cancer, Malignant peripheral nerve sheath tumour, Solid tumours, Pancreatic adenocarcinoma | (89) |
