Table 3.
Methionine adenosyltransferase II alpha inhibitors.
| Drug Name | Structure | Clinical trial identifier (if applicable) | Clinical trial stage (if applicable) | Cancer type in trial (if applicable) |
Citation |
|---|---|---|---|---|---|
| Cycloleucine |
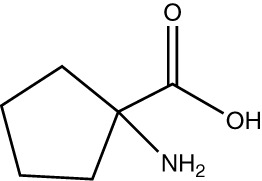
|
(91) | |||
| Aminobicyclo-hexane-carboxylic acid |
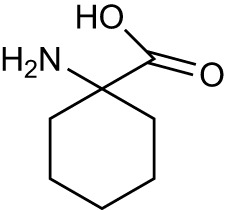
|
(92) | |||
| FIDAS agents (generic structure) - X, Y and Z are variable groups |
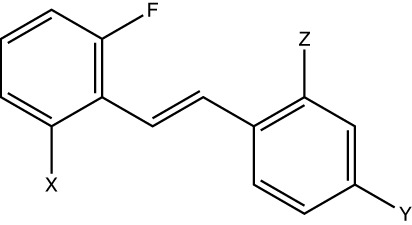
|
(93, 94) | |||
| PF-9366 |
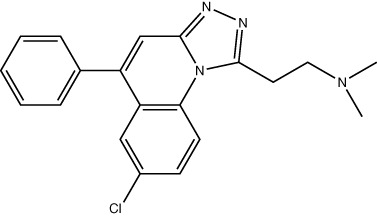
|
(95) | |||
| AGI-25696 |
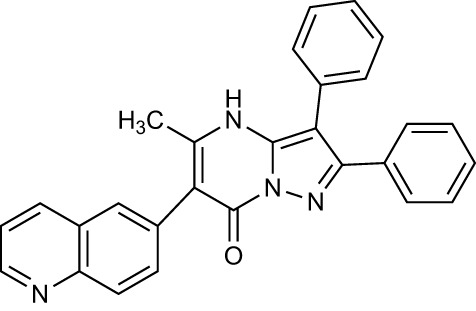
|
(96) | |||
| AG-270 (S095033) |
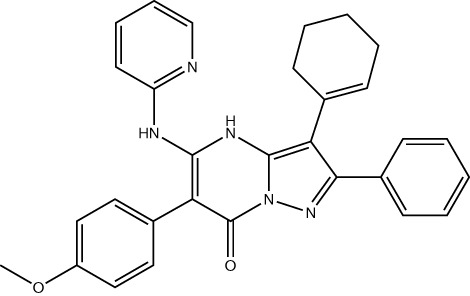
|
NCT03435250, NCT05312372 | Phase 1, Phase 1/2 |
Advanced solid tumours, Lymphoma, Oesophageal squamous cell carcinoma | (74) |
| Compound 28 |
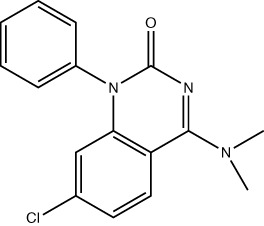
|
(97) | |||
| IDE397 | Not published | NCT04794699 | Phase 1 | Solid tumour | No publications |
