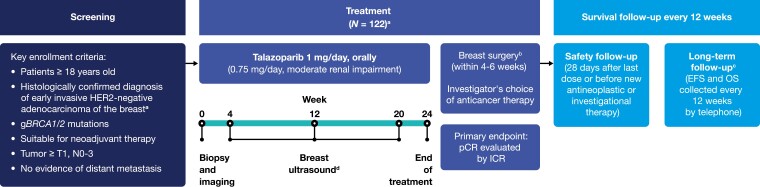
Figure 1.
Study design. Abbreviations: BC, breast cancer; EFS, event-free survival; gBRCA1/2, germline breast cancer susceptibility genes 1 and 2; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; ICR, independent central review; N, regional lymph nodes; OS, overall survival; pCR, pathologic complete response; T, primary tumor. aStudy design was amended to include HR-positive, HER2-negative patients with BC and the patient numbers were reduced from 122 to 60 to address lower than expected enrollment. bBreast/axillary tumor tissue must be removed by either lumpectomy or mastectomy with clinically appropriate axillary surgery. Patients who had disease progression were to discontinue treatment on study and switch to alternative systemic therapy or go straight to surgery. cLong-term follow-up was planned to be at least 3 years, starting from the date of surgery for EFS and after the first dose of drug for OS. However, Pfizer decided to make a strategic change in the development program for talazoparib in neoadjuvant BC and decided not to pursue further development in this setting. The study was closed after all patients completed safety follow-up, and EFS/OS was not reached. dBreast ultrasound scans at week 12 were used to assist in clinical response assessment during treatment and to provide investigator reassurance that the patient was not progressing. Breast ultrasound was not used to assess response.