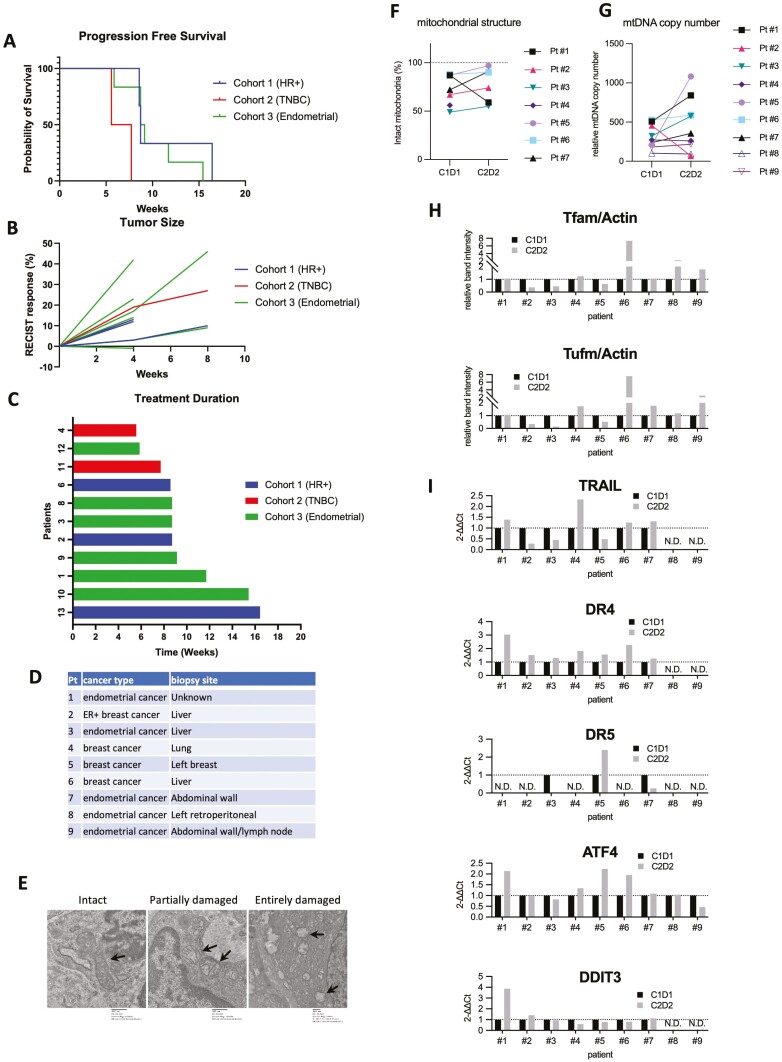
Figure 1.
(A) Progression-free survival. (B) Response by RECIST v1.1. (C) Duration of ONC201 treatment. (D) Patients whose biopsy samples compared between cycle 1 day1 and Cycle 2 Day 28. (E) Representative images showing intact, partially damaged, and severely damaged mitochondria. Intact mitochondria was from Pt #1 (cycle 1 day 1, 10000×), partially damaged mitochondria Pt #1(cycle 2 day 28, 10000×), severely damaged mitochondria Pt #3 (Cycle 1 Day 1, 5000×). (F) Mitochondrial structural analysis by electron microscopy images. (G) mtDNA copy number qPCR. (H) Immunobloting analysis of Tfam and Tufm normalized with actin as an internal control. (I) qPCR analysis of TRAIL,DR4, DR5 and ATF4, and DDIT3, normalized with GAPDH. ATF4, activating transcription factor 4; DR, death receptor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; mtDNA, mitochondrial DNA; N.D., not determined due to no detectable amplification;qPCR, quantitative polymerase chain reaction; RECIST, Response Evaluation Criteria in Solid Tumors; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.