Abstract
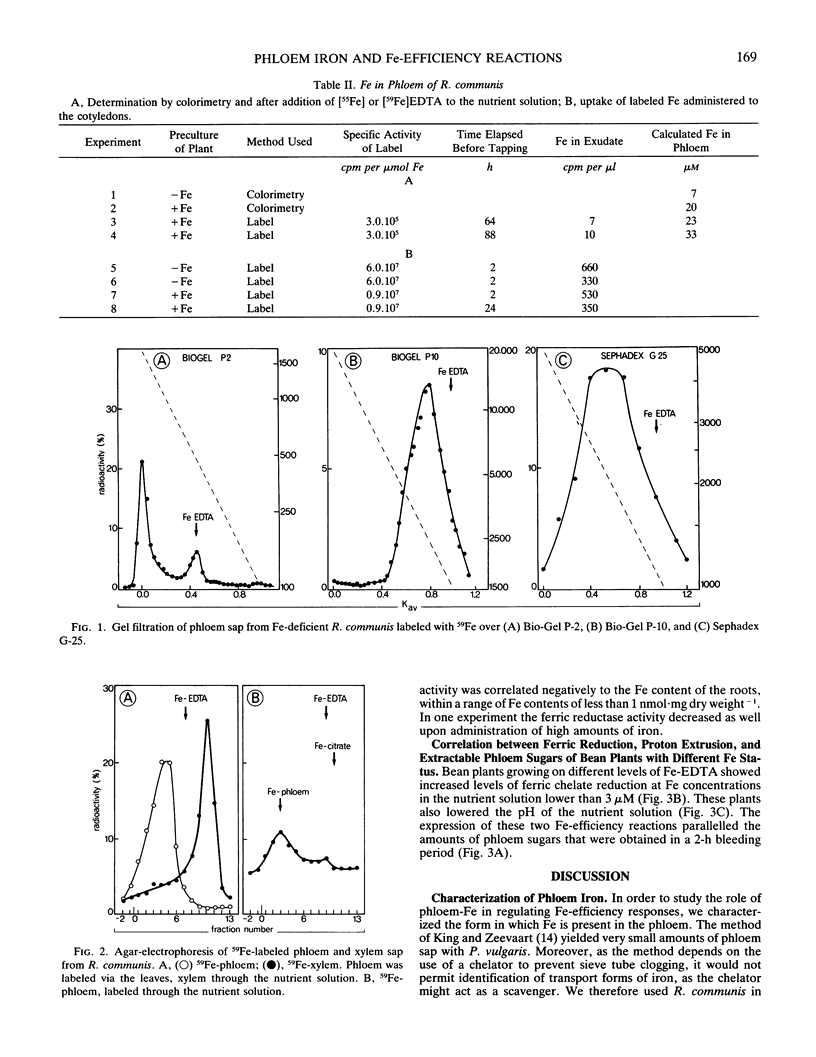
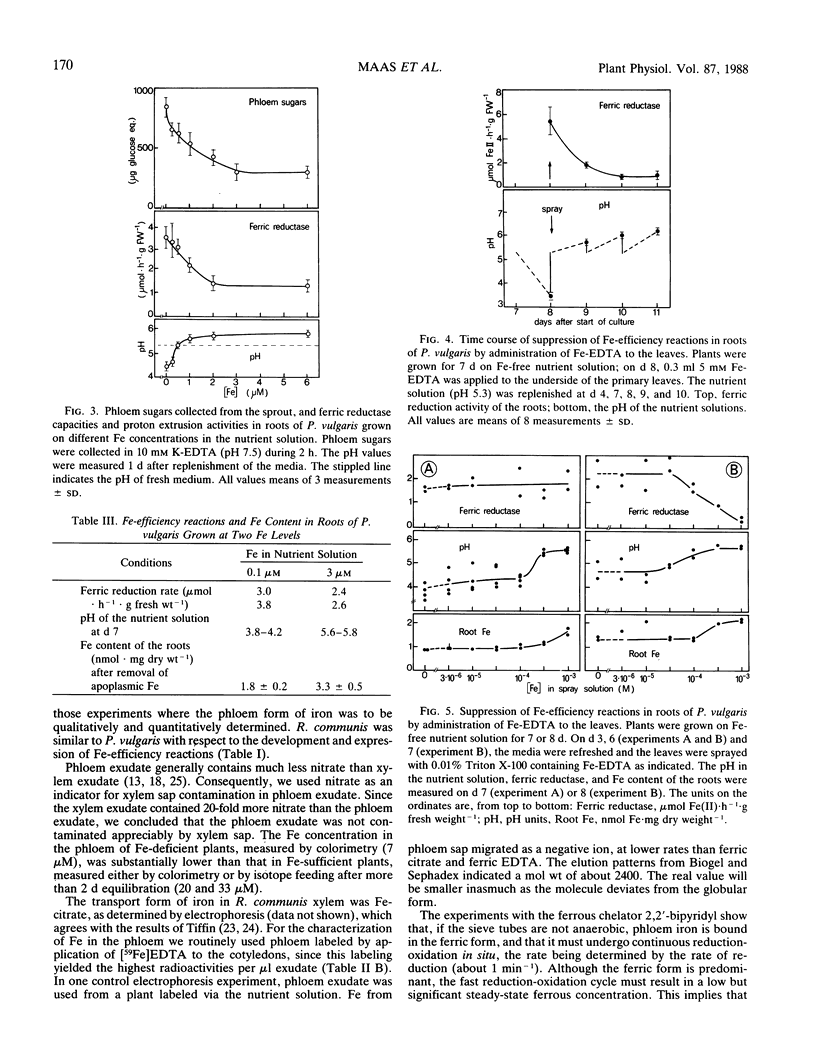
`Fe-efficiency reactions' are induced in the roots of dicotyledonous plants as a response to Fe deficiency. The role of phloem Fe in the regulation of these reactions was investigated. Iron travels in the phloem of Ricinus communis L. as a complex with an estimated molecular weight of 2400, as determined by gel exclusion chromatography. The complex is predominantly in the ferric form, but because of the presence of reducing compounds in the phloem sap, there must be a fast turnover in situ between ferric and ferrous (k ≈ 1 min−1). Iron concentrations in R. communis phloem were determined colorimetrically or after addition of 59Fe to the nutrient solution. The iron content of the phloem in Fe-deficient plants was lower (7 micromolar) than in Fe-sufficient plants (20 micromolar). Administration of Fe-EDTA to leaves of Phaseolus vulgaris L. increased the iron content of the roots within 2 days, and decreased proton extrusion and ferric chelate reduction. The increase in iron content of the roots was about the same as the difference between iron contents of roots grown on two iron levels with a concomitantly different expression of Fe-efficiency reactions. We conclude that the iron content of the leaves is reflected by the iron content of the phloem sap, and that the capacity of the phloem to carry iron to the roots is sufficient to influence the development of Fe-efficiency reactions. This does not preclude other ways for the shoot to influence these reactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienfait H. F., de Weger L. A., Kramer D. Control of the development of iron-efficiency reactions in potato as a response to iron deficiency is located in the roots. Plant Physiol. 1987 Feb;83(2):244–247. doi: 10.1104/pp.83.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienfait H. F., van den Briel W., Mesland-Mul N. T. Free space iron pools in roots: generation and mobilization. Plant Physiol. 1985 Jul;78(3):596–600. doi: 10.1104/pp.78.3.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. L., Yamaguchi S., Leal-Diaz J. Evidence for Translocation of Iron in Plants. Plant Physiol. 1965 Jan;40(1):35–38. doi: 10.1104/pp.40.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. C., Foy C. D., Bennett J. H., Christiansen M. N. Two light sources differentially affected ferric iron reduction and growth of cotton. Plant Physiol. 1979 Apr;63(4):692–695. doi: 10.1104/pp.63.4.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukovac M. J., Wittwer S. H. Absorption and Mobility of Foliar Applied Nutrients. Plant Physiol. 1957 Sep;32(5):428–435. doi: 10.1104/pp.32.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney R. L., Brown J. C., Tiffin L. O. Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol. 1972 Aug;50(2):208–213. doi: 10.1104/pp.50.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddings J. L., Brown A. L. Absorption and translocation of foliar-applied iron. Plant Physiol. 1967 Jan;42(1):15–19. doi: 10.1104/pp.42.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Zeevaart J. A. Enhancement of Phloem exudation from cut petioles by chelating agents. Plant Physiol. 1974 Jan;53(1):96–103. doi: 10.1104/pp.53.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg E. C. Function of Rhizodermal Transfer Cells in the Fe Stress Response Mechanism of Capsicum annuum L. Plant Physiol. 1986 Oct;82(2):511–517. doi: 10.1104/pp.82.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk R., Sergeant A. A simple method for dialysis of small-volume samples. Anal Biochem. 1980 Jul 1;105(2):403–404. doi: 10.1016/0003-2697(80)90477-7. [DOI] [PubMed] [Google Scholar]
- Tiffin L. O. Iron Translocation II. Citrate/Iron Ratios in Plant Stem Exudates. Plant Physiol. 1966 Mar;41(3):515–518. doi: 10.1104/pp.41.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin L. O. Iron translocation I. Plant culture, exudate sampling, iron-citrate analysis. Plant Physiol. 1966 Mar;41(3):510–514. doi: 10.1104/pp.41.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos C. R., Lubberding H. J., Bienfait H. F. Rhizosphere acidification as a response to iron deficiency in bean plants. Plant Physiol. 1986 Jul;81(3):842–846. doi: 10.1104/pp.81.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beusichem M. L., Baas R., Kirkby E. A., Nelemans J. A. Intracellular pH Regulation during NO(3) Assimilation in Shoot and Roots of Ricinus communis. Plant Physiol. 1985 Aug;78(4):768–773. doi: 10.1104/pp.78.4.768. [DOI] [PMC free article] [PubMed] [Google Scholar]


