Abstract
Background
Data on the use of the wearable cardioverter‐defibrillator (WCD) among patients with myocarditis remain sparse. Consequently, evidence for guideline recommendations in this patient population is lacking.
Methods and Results
In total, 1596 consecutive patients were included in a multicenter registry from 8 European centers, with 124 patients (8%) having received the WCD due to myocarditis and reduced left ventricular ejection fraction or prior ventricular tachyarrhythmia. The mean age was 51.6±16.3 years, with 74% being male. Patients were discharged after index hospitalization on heart failure medication: Angiotensin‐converting enzyme inhibitors (62.5%), angiotensin‐receptor‐neprilysin inhibitor (22.9%), aldosterone‐antagonists (51%), or beta blockers (91.4%). The initial median left ventricular ejection fraction was 30% (22%–45%) and increased to 48% (39%–55%) over long‐term follow‐up (P<0.001). The median BNP (brain natriuretic peptide) level at baseline was 1702 pg/mL (565–3748) and decreased to 188 pg/mL (26–348) over long‐term follow‐up (P=0.022). The mean wear time was 79.7±52.1 days and 21.0±4.9 hours per day. Arrhythmic event rates documented by the WCD were 9.7% for nonsustained ventricular tachycardia, 6.5% for sustained ventricular tachycardia, and 0% for ventricular fibrillation. Subsequently, 2.4% of patients experienced an appropriate WCD shock. The rate of inappropriate WCD shocks was 0.8%. All 3 patients with appropriate WCD shock had experienced ventricular tachycardia/ventricular fibrillation before WCD prescription, with only 1 patient showing a left ventricular ejection fraction <35%.
Conclusions
Patients with myocarditis and risk for occurrence of ventricular tachyarrhythmia may benefit from WCD use. Prior ventricular arrhythmia might appear as a better risk predictor than a reduced left ventricular ejection fraction <35% in this population.
Keywords: myocarditis, sudden cardiac death, ventricular tachycardia, wearable cardioverter‐defibrillator
Subject Categories: Arrhythmias, Sudden Cardiac Death
Nonstandard Abbreviations and Acronyms
- LGE
late gadolinium enhancement
- WCD
wearable cardioverter‐defibrillator
Clinical Perspective.
What Is New?
The wearable cardioverter‐defibrillator is frequently used in patients with myocarditis with reduced left ventricular ejection fraction or following ventricular tachyarrhythmia.
Prior ventricular tachyarrhythmia appears more important in prediction of future life‐threatening arrhythmic events compared with a reduced left ventricular ejection fraction <35%.
What Are the Clinical Implications?
Ventricular tachyarrhythmia during the acute index presentation should be incorporated during evaluation of wearable cardioverter‐defibrillator and possibly even permanent implantable cardioverter‐defibrillator treatment.
Myocarditis is an inflammatory disease of the heart muscle following exposure to an infectious agent (mainly viruses), toxic substances, drugs (eg, eosinophilic myocarditis), or developing due to dysregulations in the immune system. 1 The clinical presentation varies broadly, ranging from asymptomatic cases over heart failure symptoms or brady‐ and tachyarrhythmias up to fulminant cases with cardiogenic shock and need for mechanical circulatory support. 1 , 2 Ventricular tachyarrhythmias such as ventricular tachycardia (VT) or fibrillation (VF) can occur during the acute (hot) phase of ongoing inflammation as well as the (cold) phase of chronic or previous myocarditis (resembling the phenotype of nonischemic cardiomyopathy), entailing a substantial risk for sudden cardiac death. 2 Kragholm et al observed a relevant increase in mortality, heart failure, and VT/VF/cardiac arrest in the first 90 days following an initial hospitalization for myocarditis. 3
Whether malignant arrhythmias during the hot phase are of truly transient nature and disappear with myocardial healing remains a matter of debate and, therefore, guideline recommendations are not clear in this scenario. Furthermore, differences between individual pathologies exist, with giant cell myocarditis bearing the highest arrhythmic risk. 4 Rosier et al describe a very high rate of ventricular tachyarrhythmias (39% during 3 years of follow‐up) in patients having received an implantable cardioverter‐defibrillator (ICD) due to acute myocarditis complicated by VT/VF. 5 The novel 2022 guidelines of the European Society of Cardiology give a IIa C indication for implantation of an ICD in patients with acute myocarditis and unstable VT or VF before hospital discharge. 4 The 2017 guidelines of the American Heart Association state a IIb C indication for an ICD only in patients with giant cell myocarditis and unstable VT/VF under guideline‐directed medical therapy. 6
Over the past years the wearable cardioverter‐defibrillator (WCD) has become a valuable tool for protecting patients with a possibly just transiently increased risk for sudden cardiac death. 7 , 8 , 9 , 10 Unfortunately, despite representing a frequent indication for WCD prescription (9.8% in the study by Wäßnig et al 7 ), detailed data on patients with myocarditis and WCD are lacking and mainly single‐center experiences. 11 , 12 The 2015 European Society of Cardiology 13 and 2017 American Heart Association 6 guidelines recommended a WCD in patients with active myocarditis and poor left ventricular ejection fraction (LVEF) with a IIb C and IIb B indication, respectively. However, the novel 2022 European Society of Cardiology guidelines give no explicit recommendation in myocarditis due to sparse data. 4 According to the working group of electrophysiology and rhythmology (German Electrophysiology Working Group) of the German Cardiac Society, a WCD in acute myocarditis should be considered if LVEF <35%, nonsustained VT/VF, relevant scarring in cardiac magnetic resonance imaging (CMR), or high‐risk conditions such as giant cell myocarditis. 14
We therefore sought to investigate patients with myocarditis treated with a WCD in a multicenter registry.
METHODS
Patient Recruitment
The multicenter registry collected data of 1596 patients with prescription of a WCD (LifeVest; ZOLL, Pittsburgh, PA) between April 2012 and December 2021 from 8 hospitals in Germany and Switzerland (Bergmannsheil University Hospital, University Hospital Zurich, University Hospital Mannheim, Helios Clinic Krefeld, University Hospital St. Josef‐Hospital Bochum, Klinikum St. Georg Leipzig, University Hospital Bonn, Frankfurt University Hospital). 15 The study was approved by the appropriate local ethics committees. Due to the retrospective nature of the study, informed consent was waived by the ethics committees. All procedures, analyses, and statistical evaluations were performed in accordance with guidelines and regulations of the institutional research committee and conform to the 1975 Declaration of Helsinki.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data Collection From Wearable Cardioverter‐Defibrillator
The WCD was programmed as described previously, 15 and data were extracted from ZOLL LifeVest Network. 10 , 15 Independent physicians reviewed and classified individual arrhythmic events that were recorded by the WCD. VT episodes were graded as sustained (lasting ≥30 seconds) or nonsustained (lasting <30 seconds). WCD shocks were graded as appropriate (shock for VT or VF) or inappropriate (shock for nonventricular tachyarrhythmia).
Clinical Data Collection
All data were retrospectively collected from the electronic patient records. Evaluation included comorbidities, discharge medication, and ECG and echocardiography parameters. In addition to baseline parameters at initial hospital stay when WCD was prescribed, data were also evaluated at 3 months (short term) of follow‐up and at 6 to 12 months (long term) if possible. LVEF was calculated from transthoracic echocardiography using the biplane Simpson's method. Reasons for termination of WCD use, possible ICD implantation, and hospitalizations were additionally screened. The indication for prolongation or early termination of WCD use was at the discretion of the treating physician and was handled mainly based on changes in LVEF or occurrence of arrhythmic events. In patients with ICD implantation following WCD use, data from device interrogations were additionally retrieved if possible.
Statistical Analysis
Continuous variables were analyzed for normal distribution using the Shapiro–Wilk normality test. Data are presented as mean±SD if normally and as median with 25th and 75th percentile if nonnormally distributed. Categorical variables are displayed as frequencies and percentages. LVEF and BNP (brain natriuretic peptide) were compared using a linear mixed effect model. A 2‐sided P value <0.05 was considered statistically significant. IBM SPSS software (Version 25.0, IBM Corp., Armonk, NY) and GraphPad Prism (Version 5.03, GraphPad Software, San Diego, CA) were used for statistical analyses.
RESULTS
Baseline Characteristics and Clinical Follow‐Up of Patient Cohort With Myocarditis
In total, 1596 consecutive patients at 8 European centers were retrospectively included into the registry following WCD prescription. In 124 patients (8%), the indication for WCD was myocarditis with reduced LVEF or documented ventricular tachyarrhythmia (Figure 1). A total of 40% of patients had experienced VT/VF before WCD use (secondary prevention). Patients were predominantly male (74.2%) and had a mean age of 51.6±16.3 years (Table 1). Main comorbidities were smoking (41.2%), arterial hypertension (38%), hyperlipidemia (32.3%), coronary artery disease (24.7%), atrial fibrillation/atrial flutter (18.9%), and diabetes (16.5%). CMR revealed late gadolinium enhancement (LGE) in 91.6% of patients. Mean duration of index hospitalization was 16.3±13.7 days. Patients were discharged on a balanced heart failure medication: Angiotensin‐converting enzyme inhibitors (62.5%), angiotensin‐receptor‐neprilysin inhibitors (22.9%), aldosterone antagonists (51%), and beta blockers (91.4%).
Figure 1. WCD indication.
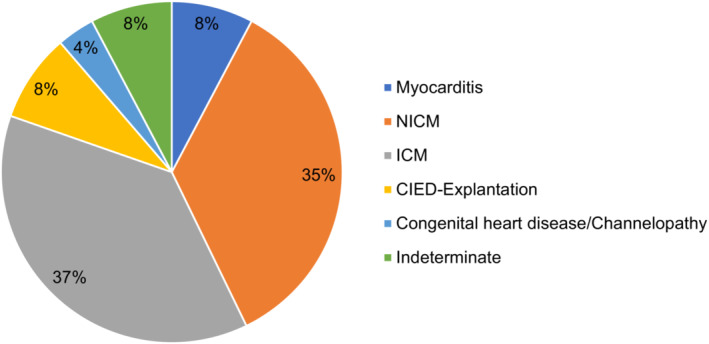
Distribution of individual indications for use of the WCD. CIED indicates cardiac implantable electronic device; ICM, ischemic cardiomyopathy; NICM, nonischemic cardiomyopathy; and WCD, wearable cardioverter‐defibrillator.
Table 1.
Baseline Characteristics
| Variables | Myocarditis (n=124) |
|---|---|
| Demographics | |
| Male sex | 92/124 (74.2) |
| Age, y | 51.6±16.3 |
| Comorbidities | |
| Coronary artery disease | 23/93 (24.7) |
| Myocardial infarction | 6/96 (6.25) |
| Coronary artery bypass graft | 0/95 (0) |
| Chronic obstructive pulmonary disease | 8/93 (8.6) |
| Chronic kidney disease/dialysis | 9/93 (9.7) |
| Atrial fibrillation or atrial flutter | 21/111 (18.9) |
| Transient ischemic attack/stroke | 7/93 (7.5) |
| Diabetes | 16/97 (16.5) |
| Smoker | 40/97 (41.2) |
| Hypertension | 38/100 (38) |
| Hhyperlipidemia | 30/93 (32.3) |
| Body mass index, kg/m2 | 27.7±5.6 |
| Hospitalization | |
| Cardiogenic shock at diagnosis | 23/93 (24.7) |
| Pulmonary edema | 11/93 (11.8) |
| Duration of hospitalization, d | 16.3±13.7 |
| Drug treatment | |
| Angiotensin‐converting enzyme inhibitors | 60/96 (62.5) |
| Angiotensin‐receptor‐neprilysin inhibitors | 22/96 (22.9) |
| Aldosterone antagonists | 49/96 (51) |
| Beta blockers | 106/116 (91.4) |
| Amiodarone | 13/117 (11.1) |
| Clinical imaging results | |
| Magnetic resonance imaging | 98/105 (93.3) |
| Late gadolinium enhancement | 87/95 (91.6) |
| LVEF | |
| LVEF at baseline, % | 30 (22–45) |
| LVEF short‐term, % | 44 (30–53) |
| LVEF long‐term, % | 48 (39–55) |
| Final EF >35% | 93/112 (83) |
| Changes in LVEF | |
| No change | 22/112 (19.6) |
| Increase in 3 mo | 58/112 (51.8) |
| Increase in 6–12 mo | 23/112 (20.5) |
| Decline in LVEF | 9/112 (8) |
| BNP | |
| BNP at baseline, pg/mL | 1702 (565–3748) |
| BNP short term, pg/mL | 688 (230–1769) |
| BNP long term, pg/mL | 188 (26–348) |
Values are n (%), mean±SD or median (interquartile range, 25th–75th percentile). BNP indicates brain natriuretic peptide; and LVEF, left ventricular ejection fraction.
Median LVEF at baseline was 30% (22–45) and significantly increased over follow‐up to 48% (39–55) (P<0.001; Figure 2A; Table 1). In 51.8% of patients, LVEF increased within the first 3 months, and in an additional 20.5% of patients within 6 to 12 months, resulting in 83% of patients showing an LVEF >35% (Table 1). Baseline BNP levels decreased significantly from 1702 pg/mL (565–3748) to 188 pg/mL (26–348) (P=0.022; Figure 2B; Table 1). Median QRS duration at baseline was 100 milliseconds (94–122.5), and PQ interval was 160 milliseconds (140–180) without relevant changes over time (Table 2). The initial median heart rate‐corrected QT interval was 437 milliseconds (408–483) and shortened over time (409 milliseconds [387–445.5] at long‐term follow‐up; P<0.01).
Figure 2. Changes in LVEF and BNP.
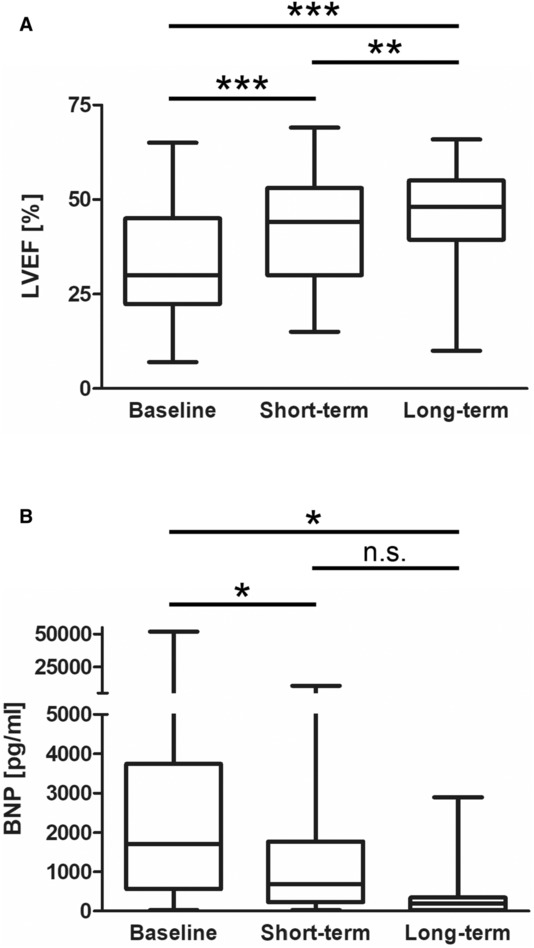
A, Box‐whisker plot of LVEF at baseline (n=124), short‐ (n=105) and long‐term (n=67) follow‐up. B, Box‐whisker plot of BNP levels at baseline (n=58), short‐ (n=35), and long‐term (n=15) follow‐up. Boxes represent median and 25th–75th percentiles. Whiskers show minimum and maximum values. For statistical comparison a linear mixed effect model was used. A 2‐sided P value <0.05 was considered statistically significant. *P<0.05; **P<0.01; ***P<0.001. BNP indicates brain natriuretic peptide; LVEF, left ventricular ejection fraction; and n.s., not significant.
Table 2.
ECG Parameters
| Variables | Myocarditis (n=124) |
|---|---|
| Left bundle‐branch block, n (%) | 23/89 (25.8) |
| Right bundle‐branch block, n (%) | 5/89 (5.6) |
| QRS at baseline, ms | 100 (94–122.5) |
| QRS short‐term, ms | 101 (93.75–120) |
| QRS long‐term, ms | 103.5 (92–121.5) |
| QTc at baseline, ms | 437 (408–483) |
| QTc short‐term, ms | 419 (387.8–453) |
| QTc long‐term, ms | 409 (387–445.5) |
| PQ at baseline, ms | 160 (140–180) |
| PQ short‐term, ms | 160 (145.5–177.5) |
| PQ long‐term, ms | 162 (150–194) |
Values are n (%) or median (interquartile range, 25th–75th percentile). QTc indicates heart rate‐corrected QT interval.
WCD Data in Patients With Myocarditis
On average the WCD was worn for 21.0±4.9 hours daily over a mean time period of 79.7±52.1 days (Table 3). A total of 36.3% of patients were treated with the WCD for more than 90 days. The compliance rate (>20 hours wear time per day) was 78.2%.
Table 3.
WCD Parameters
| Variables | Myocarditis (n=124) |
|---|---|
| Parameters of wear time | |
| Wear days | 79.7±52.1 |
| Average daily wear hours | 21.0±4.9 |
| More than 90 wear days | 45/124 (36.3) |
| Compliance (>20 h per day of wear time) | 97/124 (78.2) |
| Arrhythmic events during WCD | |
| Ventricular tachycardia | 8/124 (6.5) |
| Ventricular fibrillation | 0/124 (0) |
| Nonsustained ventricular tachycardia | 12/124 (9.7) |
| Atrial fibrillation or atrial flutter | 3/98 (3.1) |
| WCD shocks | |
| Appropriate | 3/124 (2.4) |
| Inappropriate | 1/124 (0.8) |
| Reason for stopping WCD | |
| Improved LVEF | 42/99 (42.4) |
| No further complications with normal LVEF | 10/99 (10.1) |
| Implantable cardioverter‐defibrillator/left ventricular assist device implantation or planed | 26/99 (26.3) |
| Incompliance | 4/99 (4.1) |
| Death | 2/99 (2) |
| Unknown | 13/99 (13.1) |
| Decision pending | 2/99 (2) |
Values are n (%) or mean±SD. LVEF indicates left ventricular ejection fraction; and WCD, wearable cardioverter‐defibrillator.
Sustained VT was observed in a total of 8 patients (6.5%), resulting in an appropriate WCD shock rate of 2.4% (3 patients). Nonsustained VT was seen in 12 patients (9.7%), whereas no episodes of VF were detected. Documented episodes of atrial fibrillation or atrial flutter occurred in 3.1% of patients. One young patient had an inappropriate WCD shock during rapid supraventricular tachycardia due to failure to press the response button during alarming of the WCD.
All patients with appropriate WCD shocks were female, displayed LGE on CMR, and had experienced sustained ventricular tachyarrhythmia before WCD prescription (Table 4). Only 1 patient had an LVEF <35%, which improved to 45% following cardiac resynchronization therapy. The 3 patients were finally treated with an ICD.
Table 4.
Patients With WCD Shock
| Sex | Age, y | LVEF baseline, % | LGE | Prior VT/VF | Appropriate | Arrhythmic event | ICD implantation |
|---|---|---|---|---|---|---|---|
| f | 31 | 53 | Yes | Yes | Yes | VT | Yes |
| f | 31 | 53 | Yes | Yes | Yes | VT | Planed |
| f | 51 | 30 | Yes | Yes | Yes | VT | Yes |
| f | 24 | 35 | Yes | No | No | Supraventricular tachycardia | No |
f indicates female sex; ICD, implantable cardioverter‐defibrillator; LGE, late gadolinium enhancement; LVEF, left ventricular ejection faction; VF, ventricular fibrillation; and VT, ventricular tachycardia.
For 99 patients, the reason for stopping WCD could be assessed: 42.4% of patients could stop the WCD use due to improved LVEF, whereas 26.3% received an ICD or left ventricular assist device; 4.1% of patients refused further WCD wearing; and 2 (1.6%) patients died during prescription of the WCD, but both deaths were not attributable to an arrhythmic or heart failure event.
Further Follow‐Up After WCD Use
After the end of WCD use, a total of 25 patients received an ICD (Table 5). Device interrogations during further follow‐up could be obtained from 20 of those patients and revealed sustained VT in 5 with consecutive ICD shocks in 2 patients. Nonsustained VT was detected in 7 patients, but no VF was observed.
Table 5.
Follow‐Up After End of WCD
| Variables | Myocarditis (n=124) |
|---|---|
| Cardiac implantable electronic devices | |
| Device implantation | 25/121 (20.7) |
| Planed implantation | 2/121 (1.7) |
| Died before implantation | 2/121 (1.7) |
| Patient denied | 5/121 (4.1) |
| Arrhythmic events postcardiac implantable electrical device | |
| Ventricular tachycardia | 5/20 (25) |
| Ventricular fibrillation | 0/20 (0) |
| Shock | 2/20 (10) |
| Nonsustained ventricular tachycardia | 7/20 (35) |
| Death | |
| Death during follow‐up | 7/124 (5.6) |
| Arrhythmic death | 0/4 (0) |
| Other cause of death | 1/4 (25) |
| Indetermined death | 3/4 (75) |
| Rehospitlaization | |
| Rehospitlaization | 32/93 (34.4) |
| Ventricular tachycardia/ventricular fibrillation | 2/24 (8.3) |
| Atrial fibrillation | 6/24 (25) |
| Other cardiovascular cause of rehospitalization | 9/24 (38) |
| Any other cause | 7/24 (28) |
Values are n (%). WCD indicates wearable cardioverter‐defibrillator.
Death occurred in a total of 7 patients but could not be attributed to arrhythmic events. Rehospitalizations were frequent (34.4%) and were mainly due to sustained VT/VF (8.3%), atrial fibrillation (25%), or other cardiovascular causes (38%).
DISCUSSION
In the current study we analyzed a total of 124 patients with myocarditis, which represented 8% of the complete cohort of our multicenter registry. This is comparable to the German WCD registry published in 2016 by Wäßnig et al, which reported 595 patients with myocarditis, representing 9.8% of all patients. 7 But this patient cohort was not systematically studied and analyzed because patient data were derived only from ZOLL. We observed sustained VT in 6.5% of cases, resulting in an appropriate WCD shock rate of 2.4%, which is slightly higher compared with the German WCD registry (1.3%). 7 Two smaller single‐center studies with 39 12 and 59 11 patients reported VT in 17.9% and 3.4% of patients, with consecutive shock rates of 2.6% and 0%, respectively. Similar to our results, no episodes of VF were reported. Other registries with large patient numbers like WEARIT‐II 9 (Prospective Registry of Patients Using the WCD) or WEARIT‐II‐Europe 8 had no information on myocarditis or included myocarditis cases within the subgroup of nonischemic cardiomyopathy, respectively. Importantly, detailed patient‐level data on, for example CMR, LGE, or medications were not readily available in the bigger studies. Taken together, data on use of the WCD in myocarditis are scarce, and our cohort adds to the evidence that ventricular tachyarrhythmia can be effectively treated by the WCD in these patients.
Predicting or identifying patients with myocarditis at risk for sudden cardiac death due to VT/VF is challenging for the treating physician. Parameters such as QRS duration, heart rate‐corrected QT interval prolongation, LGE in CMR, severely reduced LVEF, or frequent nonsustained VTs have been proposed as markers of increased risk. 16 The WCD prescription in patients with myocarditis reported in our cohort appears rather adequate and not too liberal regarding a median LVEF of 30% as well as high rates of LGE (91.6%) and secondary preventive indications (40%). The heart rate‐corrected QT interval was prolonged in our patient cohort, which represents a frequent finding in myocarditis, 17 , 18 but normalized over time. Unfortunately, the event rate was too low for adequate assessment of predictors of WCD shocks.
Whether the occurrence and risk of recurrence of ventricular tachyarrhythmias during the acute (hot) phase truly dissolves after myocardial healing remains unclear. An initial severe reduction in LVEF as at least partial possible mediator of the increased risk of sudden cardiac death often improves during the healing process, as observed in our study. Interestingly, all 3 patients who experienced a WCD shock had VT/VF events before WCD prescription, but only 1 patient had a severely reduced LVEF. Therefore, prior ventricular arrhythmia might appear as a better risk predictor than a reduced LVEF <35% in the acute setting. Additionally, 1 study even describes a substantial rate of VT/VF occurring in more than every third patient within 3 years after ICD implantation due to acute myocarditis complicated by ventricular tachyarrhythmia. 5 Our study includes device interrogations of 20 patients having received an ICD after end of WCD use and reveals VT/VF in 25% and ICD shocks in 10% of patients, demonstrating a thorough patient selection. One patient with ICD shock had VT before and during WCD, and the other patient had a nonsustained VT documented by the WCD and a reduced LVEF. The observed increased risk of patients with prior ventricular arrhythmia for WCD and also later ICD shocks might support the novel IIa C recommendation for implantation of an ICD in patients with acute myocarditis and unstable VT or VF before hospital discharge proposed by the 2022 guidelines of the European Society of Cardiology, 4 as these patients bear a substantial risk for ventricular tachyarrhythmia possibly not confined to the initial inflammatory phase. Relying only on LVEF during evaluation of WCD or ICD treatment appears insufficient when assessing the risk for life‐threatening arrhythmic events in individual patients with myocarditis. For patients with less defined (high‐)risk criteria, the WCD offers appropriate protection from sudden cardiac death and might prevent early and potentially unnecessary ICD implantations. The thorough rhythm monitoring granted by the WCD could add valuable information in ambiguous situations following WCD use.
Study Limitations
As a major limitation it has to be acknowledged that the presented registry data are of retrospective nature and primarily show what clinicians are doing. No causal claims can be derived from this study. Although the study reports more detailed data than larger registries, no further details on myocarditis (eg, location of LGE, biopsy results) were obtained. As indicated in the respective tables, individual patient data were not always available for every parameter.
CONCLUSIONS
The WCD is frequently used in patients with myocarditis and reduced LVEF or prior ventricular tachyarrhythmia and can adequately terminate life‐threatening ventricular arrhythmias. WCD treatment seems most appropriate in patients with VT/VF during the acute index presentation, whereas a reduced LVEF <35% appears a weaker risk predictor. Probably also during evaluation of permanent ICD implantation, prior ventricular arrhythmia should be incorporated or even prioritized over LVEF. Randomized controlled trials on WCD treatment in myocarditis are needed to assess its impact on hard outcomes such as mortality and sudden cardiac death but unfortunately are unlikely to be performed.
Sources of Funding
We acknowledge support by the Open Access Publication Funds of the Ruhr‐Universität Bochum.
Disclosures
None.
This article was sent to Sakima A. Smith, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 7.
Contributor Information
Ibrahim El‐Battrawy, Email: ibrahim.elbattrawy2006@gmail.com.
Thomas Beiert, Email: thomas.beiert@ukbonn.de.
References
- 1. Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, Friedrich MG, Klingel K, Lehtonen J, Moslehi JJ, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy. Circulation Hear Fail. 2020;13:e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peretto G, Sala S, Rizzo S, Luca GD, Campochiaro C, Sartorelli S, Benedetti G, Palmisano A, Esposito A, Tresoldi M, et al. Arrhythmias in myocarditis: state of the art. Heart Rhythm. 2019;16:793–801. doi: 10.1016/j.hrthm.2018.11.024 [DOI] [PubMed] [Google Scholar]
- 3. Kragholm KH, Lindgren FL, Zaremba T, Freeman P, Andersen NH, Riahi S, Pareek M, Køber L, Torp‐Pedersen C, Søgaard P, et al. Mortality and ventricular arrhythmia after acute myocarditis: a nationwide registry‐based follow‐up study. Open Heart. 2021;8:e001806. doi: 10.1136/openhrt-2021-001806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeppenfeld K, Tfelt‐Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, Charron P, Corrado D, Dagres N, Chillou C, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43:3997–4126. doi: 10.1093/eurheartj/ehac262 [DOI] [PubMed] [Google Scholar]
- 5. Rosier L, Zouaghi A, Barré V, Martins R, Probst V, Marijon E, Sadoul N, Chauveau S, Costa AD, Badoz M, et al. High risk of sustained ventricular arrhythmia recurrence after acute myocarditis. J Clin Med. 2020;9:848. doi: 10.3390/jcm9030848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al‐Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018;138:e272–e391. doi: 10.1161/CIR.0000000000000549 [DOI] [PubMed] [Google Scholar]
- 7. Wäßnig NK, Günther M, Quick S, Pfluecke C, Rottstädt F, Szymkiewicz SJ, Ringquist S, Strasser RH, Speiser U. Experience with the wearable cardioverter‐defibrillator in patients at high risk for sudden cardiac death. Circulation. 2016;134:635–643. doi: 10.1161/CIRCULATIONAHA.115.019124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Veltmann C, Winter S, Duncker D, Jungbauer CG, Wäßnig NK, Geller JC, Erath JW, Goeing O, Perings C, Ulbrich M, et al. Protected risk stratification with the wearable cardioverter‐defibrillator: results from the WEARIT‐II‐EUROPE registry. Clin Res Cardiol. 2021;110:102–113. doi: 10.1007/s00392-020-01657-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kutyifa V, Moss AJ, Klein H, Biton Y, McNitt S, MacKecknie B, Zareba W, Goldenberg I. Use of the wearable cardioverter defibrillator in high‐risk cardiac patients. Circulation. 2015;132:1613–1619. doi: 10.1161/CIRCULATIONAHA.115.015677 [DOI] [PubMed] [Google Scholar]
- 10. Beiert T, Malotki R, Kraemer N, Stöckigt F, Linhart M, Nickenig G, Schrickel JW, Andrié RP. A real world wearable cardioverter defibrillator experience—very high appropriate shock rate in ischemic cardiomyopathy patients at a European single‐center. J Electrocardiol. 2017;50:603–609. doi: 10.1016/j.jelectrocard.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 11. Tscholl V, Wielander D, Kelch F, Stroux A, Attanasio P, Tschöpe C, Landmesser U, Roser M, Huemer M, Heidecker B, et al. Benefit of a wearable cardioverter defibrillator for detection and therapy of arrhythmias in patients with myocarditis. Esc Hear Fail. 2021;8:2428–2437. doi: 10.1002/ehf2.13353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blaschke F, Lacour P, Dang PL, Parwani AS, Hohendanner F, Walter T, Klingel K, Kühl U, Heinzel FR, Sherif M, et al. Wearable cardioverter‐defibrillator: friend or foe in suspected myocarditis? ESC Heart Fail. 2021;8:2591–2596. doi: 10.1002/ehf2.13340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Priori SG, Blomström‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. The task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316 [DOI] [PubMed] [Google Scholar]
- 14. Deneke T, Bosch R, Eckardt L, Nowak B, Schwab JO, Sommer P, Veltmann C, Helms TM. Der tragbare Kardioverter/Defibrillator (WCD)—Indikationen und Einsatz. Der Kardiologe. 2019;13:292–304. doi: 10.1007/s12181-019-0331-4 [DOI] [Google Scholar]
- 15. El‐Battrawy I, Kovacs B, Dreher TC, Klein N, Rosenkaimer S, Röger S, Kuschyk J, Saguner AM, Kowitz J, Erath JW, et al. Real life experience with the wearable cardioverter‐defibrillator in an international multicenter registry. Sci Rep‐UK. 2022;12:3203. doi: 10.1038/s41598-022-06007-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peretto G, Sala S, Rizzo S, Palmisano A, Esposito A, Cobelli FD, Campochiaro C, Luca GD, Foppoli L, Dagna L, et al. Ventricular arrhythmias in myocarditis characterization and relationships with myocardial inflammation. J Am Coll Cardiol. 2020;75:1046–1057. doi: 10.1016/j.jacc.2020.01.036 [DOI] [PubMed] [Google Scholar]
- 17. Lazzerini PE, Capecchi PL, Laghi‐Pasini F. Long QT syndrome: an emerging role for inflammation and immunity. Front Cardiovasc Med. 2015;2:26. doi: 10.3389/fcvm.2015.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hung Y, Lin W‐H, Lin C‐S, Cheng S‐M, Tsai T‐N, Yang S‐P, Lin W‐Y. The prognostic role of QTc interval in acute myocarditis. Acta Cardiol Sin. 2016;32:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]


