Abstract
Background
Many prenatal factors are reported to be associated with congenital heart defects (CHD) in offspring. However, these associations have not been adequately examined using large‐scale birth cohorts.
Methods and Results
We evaluated a data set of the Japan Environmental and Children's Study. The primary outcome was a diagnosis of CHD by age 2 years. We defined the following variables as exposures: maternal baseline characteristics, fertilization treatment, maternal history of diseases, socioeconomic status, maternal alcohol intake, smoking, tea consumption, maternal dietary intake, and maternal medications and supplements up to 12 weeks of gestation. We used multivariable logistic regression analysis to assess the associations between various exposures and CHD in offspring. A total of 91 664 singletons were included, among which 1264 (1.38%) had CHD. In multivariable analysis, vitamin A supplements (adjusted odds ratio [aOR], 5.78 [95% CI, 2.30–14.51]), maternal use of valproic acid (aOR, 4.86 [95% CI, 1.51–15.64]), maternal use of antihypertensive agents (aOR, 3.80 [95% CI, 1.74–8.29]), maternal age ≥40 years (aOR, 1.59 [95% CI, 1.14–2.20]), and high maternal hemoglobin concentration in the second trimester (aOR, 1.10 per g/dL [95% CI, 1.03–1.17]) were associated with CHD in offspring.
Conclusions
Using a Japanese large‐scale birth cohort study, we found 6 maternal factors to be associated with CHD in offspring.
Keywords: birth cohort, congenital heart defect, environmental factor, maternal factor, Japan Environment and Children's Study
Subject Categories: Etiology, Pediatrics, Risk Factors, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- CHD
congenital heart defects
- JECS
Japan Environment and Children's Study
Clinical Perspective.
What Is New?
This was an exploratory study of the association between congenital heart defects in offspring and prenatal exposures using data from a large birth cohort study.
Age at pregnancy, past history of maternal congenital heart defects, higher maternal hemoglobin concentration, maternal intake of valproic acid or antihypertensive medication, and vitamin A supplementation were significant risk factors associated with congenital heart defects in offspring.
Parental socioeconomic status, maternal dietary intake, and maternal lifestyle habits did not show a significant association with congenital heart defects in offspring.
What Are the Clinical Implications?
This study strengthens the evidence regarding the association of maternal factors and the risk of congenital heart defects in offspring.
To reduce the risk of congenital heart defects in offspring, further education regarding the avoidance of teratogenic medicines and supplements whenever possible is warranted for women who wish to become pregnant.
Congenital heart defects (CHD) are the most common congenital malformation, occurring in ≈1% of births. 1 Although the mortality rate has been declining because of advances in medical treatment and surgical procedures, CHD remain the leading cause of infantile death in developed countries. 1 Genetic abnormalities can cause CHD; however, a previous study identified the contribution of genetic abnormalities to the development of CHD in 45% of patients with CHD. 2 CHD can also be caused by multiple factors other than genetic abnormalities alone, including environmental factors.
Previous retrospective epidemiological studies 3 , 4 , 5 , 6 , 7 , 8 , 9 have reported that rubella, maternal intake of medications or supplements (eg, thalidomide, retinoic acid, valproic acid, lithium, calcium channel blockers, angiotensin‐converting enzyme inhibitors, vitamin A supplementation) or alcohol can cause CHD in offspring. Similarly, pregnant women with older age, obesity, diabetes, or a past history of CHD are considered to be at high risk of having offspring with CHD. 10 , 11 Four large‐scale birth cohorts, namely, the JECS (Japan Environment and Children's Study) 12 , 13 ; the Hokkaido Study of Environment and Children's Health 14 ; the Norwegian Mother, Father and Child Cohort Study 15 , 16 ; and the Danish National Birth Cohort 16 , 17 , 18 , 19 , 20 have revealed associations between CHD in offspring and maternal alcohol intake, maternal sugar load, maternal exposure to organic solvents, and maternal folic acid intake. Although these birth cohort studies have demonstrated highly reliable associations between environmental factors and CHD in offspring, the studies were designed to investigate the effects of a single exposure on multiple outcomes, including CHD in offspring. To date, no large birth cohort studies have explored the association between a variety of environmental or clinical factors that affect pregnant women and CHD in offspring, although retrospective studies such as case–control design have examined such associations. The JECS is a large‐scale birth cohort study that has enrolled >100 000 pregnant women and their offspring in Japan. Maternal factors, including parental background, dietary intake, medications, and supplementation during pregnancy, have been precisely investigated in the JECS, together with CHD in offspring.
The objective of the present study was to explore early prenatal exposure factors associated with CHD in offspring using data from the JECS study.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design, Setting, and Participants
In this study, we used data from the JECS, a prospective birth cohort study conducted in 21 locations throughout Japan. Urbanization and land development were diverse in the study area, from urban and suburban to rural areas as well as from agricultural and fishery to commercial and industrial uses. Details of the JECS study design have been published in a previous report. 21 In this study, we used the data set “jecs‐ta‐20 190 930,” released in October 2019 and updated on June 29, 2021, which contains information on 92 944 infants and their mothers recruited for the JECS between January 2011 and March 2014 from early pregnancy to age 3 years. This data set includes data on children and the environment obtained using self‐administered questionnaires completed by caregivers and data recorded at the time of delivery by a physician. We did not use information obtained from fathers because ≈50% of fathers did not complete the questionnaires. Participants who did not complete all follow‐up surveys or exposure surveys were excluded from the present study. All participants provided their written informed consent. The JECS protocol was reviewed and approved by the Ministry of the Environment's Institutional Review Board on Epidemiological Studies and the Ethics Committees of all participating institutions.
Outcomes
The primary outcome was CHD in offspring. We defined a child as having CHD if physicians reported at birth, 1 month, 6 months, age 1 year, or 2 years that the child had CHD in the questionnaires. Children with the following conditions were excluded: (1) patent ductus arteriosus in premature infants at <37 weeks of gestation; (2) spontaneous closure of patent ductus arteriosus or atrial septal defect present until 2 years; (3) improved pulmonary artery stenosis by age 2 years; (4) cardiovascular structural abnormalities without any hemodynamic abnormalities (eg, persistent left superior vena cava, interrupted inferior vena cava, right‐sided aortic arch, vascular ring, right thoracic heart, and aortic bicuspid valve without clinically significant hemodynamic abnormalities); and (5) cardiac diseases other than CHD (eg, arrhythmia, cardiomyopathy, cardiac tumor, and Kawasaki disease). According to CHD type, we classified CHD into 3 categories (simple, complex, or unknown). Complex CHD included heterotaxia, hypoplastic left heart syndrome, tricuspid valve atresia, pulmonary atresia, single ventricle, transposition of the great arteries, truncus arteriosus, double outlet of right ventricle, tetralogy of Fallot, total anomalous pulmonary venous connection, interruption or coarctation of the aorta complex, complete atrioventricular septal defect, and Ebstein disease. Other CHDs, including incomplete atrioventricular septal defect, ventricular septal defect, patent ductus arteriosus, atrial septal defect, aortic stenosis, and pulmonary stenosis, were classified as simple CHD. Cases with both complex and simple lesions were classified as complex CHD. Any CHD for which the diagnosis was not classified as simple or complex were classified as unknown CHD.
Exposures
All data for exposures were obtained during the first and second trimester of pregnancy. We defined the following variables as exposures: maternal baseline characteristics (age, height, weight, body mass index collected from the maternal questionnaire; hemoglobin concentration collected from blood test around the second trimester of pregnancy); fertilization treatment; maternal history of diseases (CHD, allergic disease, immunologic disease, neurologic/psychiatric disease, gastrointestinal disease, diabetes, and renal disease); socioeconomic status (maternal and paternal highest education level, household income); maternal lifestyle habits (smoking around the time of fertilization; alcohol consumption around the time of fertilization; intake of green tea, oolong tea, black tea, or coffee). These data were collected from the maternal questionnaire; maternal dietary intake collected from food frequency questionnaire 22 (meat, seafood, vegetables, green and yellow vegetables, beans, protein, fat, carbohydrate, retinol activity equivalent, vitamin C, α tocopherol, vitamin K, folate, and calories); and maternal use of medications and supplements up to 12 weeks of gestation collected from interviews with mothers. Age was divided into 5 age groups: <25, 25 to 29, 30 to 34, 35 to 39, and ≥40 years. Body mass index was divided into 4 categories: <20, 20 to 24, 25 to 29, or ≥30 kg/m2. Fertilization treatment was categorized as no infertility treatment, infertility treatment other than in vitro fertilization, or in vitro fertilization. A maternal allergy history included bronchial asthma, allergic rhinitis/hay fever, atopic dermatitis, allergic conjunctivitis, food allergy, drug rash/drug allergy, hives, contact dermatitis, sick building syndrome, and chemical sensitivity. Immunologic diseases included collagen diseases, autoimmune diseases, systemic lupus erythematosus, rheumatoid arthritis, and other immunologic diseases. Neurologic/psychiatric disease included depression, autonomic nervous system disorders, anxiety disorders, schizophrenia, and epilepsy. Gastrointestinal diseases included Crohn disease and ulcerative colitis. Diabetes included type 1 diabetes, type 2 diabetes, gestational diabetes, hyperthyroidism and Graves' disease, hypothyroidism and Hashimoto disease, and other endocrine system diseases. Renal diseases included chronic nephritis and nephrotic syndrome. Maternal and paternal education levels were divided into junior high or high school graduate, technical junior college or technical/vocational, and college graduate or associate degree or higher. Household income was divided into <2 million JPY, 2 to <4 million JPY, 4 to <6 million JPY, 6 to <10 million JPY, and ≥10 million JPY. Smoking around the time of fertilization was classified as smoking during pregnancy if the respondents reported “Currently smoking” or “Previously did, but quit after realizing current pregnancy”; no smoking during pregnancy was defined as respondents reporting “Never” or “Previously did, but quit before realizing current pregnancy.” Alcohol consumption around the time of fertilization was classified as current drinking if the respondent was still drinking and no current drinking if the respondent reported never drinking or used to drink but stopped. Regarding intakes of green tea, oolong tea, black tea, and coffee, the scores were as follows: 0 points for <1 cup a week; 1 for 1 to 2 cups a week, 3 for 3 to 4 cups a week, 5 for 5 to 6 cups a week, 7 for 1 cup daily, 14 for 2 to 3 cups daily, 28 for 4 to 6 cups daily, 49 for 7 to 9 cups daily, and 70 points for 10 or more cups daily. All variables for maternal dietary intake were divided into quartile ranges, with Q1, Q2, Q3, and Q4 from lowest to highest.
Statistical Analysis
All statistical analyses were conducted using R version 4.1.3. The significance level for all tests was set at 5% and the confidence coefficient for all interval estimations was 95%. All analyses were complete case analyses excluding missing data. The number and mean±SD were calculated for continuous data, and the number and proportion were calculated for categorical data. Univariable logistic regression models were used to estimate the odds ratio (OR) for each exposure variable, their Wald‐type 95% CIs and P values using the incidence of CHD as the outcome variable. Additionally, all variables with P<0.05 in the univariable logistic regression model, along with use of teratogenic drugs (antihypertensive agents, valproic acid) or supplements (retinol activity equivalent, folic acid), smoking, alcohol drinking, hemoglobin concentration, maternal history of CHD, diabetes, age, and body mass index were all included in a multivariable logistic regression model to obtain the adjusted odds ratio (aOR) for each exposure variable.
We performed sensitivity analyses excluding offspring with trisomy 21, 18, and 13 because these infants have a higher rate of CHD owing to genetic (ie, nonenvironmental) factors.
RESULTS
Incidence of CHD Outcomes in the Analyzed Data Set
The analyzed data set comprised 91 664 cases, excluding 6649 unregistered cases, 948 multiple births, 3521 miscarriages or stillbirths, 71 untraced cases, and 1209 with untraceable exposure information (Figure). Using the definitions prespecified before analysis, 1039 cases (1.13%) were determined to have simple CHD, 181 (0.20%) complex CHD, and 44 (0.05%) other unknown CHD. Among all CHD cases, ventricular septal defects accounted for 721 (57.0%), followed by atrial septal defects, pulmonary stenosis, tetralogy of Fallot, and interruption or coarctation of the aorta complex (Table 1).
Figure 1. Flowchart of analysis data set.
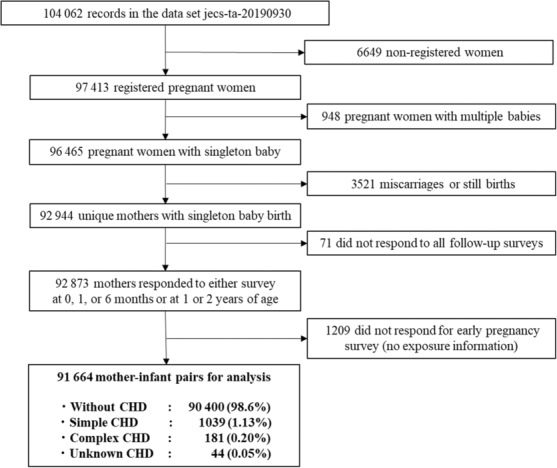
CHD indicates congenital heart defects.
Table 1.
Incidence of Congenital Heart Defects in Each Disease Category
| Frequency (%) | |
|---|---|
| All congenital heart defects | 1264 (1.38%) |
| Complex congenital heart defects | 181 (0.20%) |
| Simple congenital heart defects | 1039 (1.13%) |
| Unknown congenital heart defects | 44 (0.05%) |
| Defects | Frequency (%, among all CHD) |
| Ventricular septal defects | 721 (57.0%) |
| Atrial septal defects | 147 (11.6%) |
| Pulmonary stenosis | 93 (7.4%) |
| Patent ductus arteriosus | 54 (4.3%) |
| Tetralogy of Fallot | 34 (2.7%) |
| Interruption or coarctation of aorta complex | 28 (2.2%) |
| Atrioventricular septal defect | 22 (1.7%) |
| Truncus arteriosus | 19 (1.5%) |
| Double outlet of right ventricle | 16 (1.3%) |
| Pulmonary atresia | 13 (1.0%) |
| Single ventricle | 12 (0.95%) |
| Total anomalous pulmonary venous connection | 12 (0.95%) |
| Heterotaxia | 10 (0.79%) |
| Tricuspid valve atresia | 6 (0.47%) |
| Hypoplastic left heart syndrome | 4 (0.32%) |
| Aortic stenosis | 4 (0.32%) |
| Transposition of great arteries | 3 (0.24%) |
| Ebstein disease | 1 (0.08%) |
| Other congenital heart defects | 21 (1.66%) |
| Unknown congenital heart defects | 44 (3.48%) |
Complex CHD included heterotaxia, hypoplastic left heart syndrome, tricuspid valve atresia, pulmonary atresia, single ventricle, transposition of the great arteries, truncus arteriosus, double outlet of right ventricle, tetralogy of Fallot, total anomalous pulmonary venous connection, interruption or coarctation of the aorta complex, complete atrioventricular septal defect, and Ebstein disease. Other CHDs, including incomplete atrioventricular septal defect, ventricular septal defect, patent ductus arteriosus, atrial septal defect, aortic stenosis, and pulmonary stenosis, were classified as simple CHD. Cases with both complex and simple lesions were classified as complex CHD. Any CHD for which the diagnosis was not classified as simple or complex was classified as unknown CHD. CHD indicates congenital heart defects.
Univariable Analyses
Table 2 shows the association between the background characteristics of parents and infants with or without CHD. Maternal age ≥40 years at pregnancy (OR, 1.81 [95% CI, 1.35–2.41), hemoglobin concentration (OR, 1.10 [95% CI 1.03–1.17), in vitro fertilization (OR, 1.38 [95% CI, 1.05–1.82]), and maternal history of CHD (OR, 3.64 [95% CI, 2.12–6.25]) were positively associated with CHD; other specific factors such as smoking and alcohol consumption showed no association. Table 3 shows the association between maternal dietary intake and CHD in the offspring. There were no significant differences between the groups with and without CHD for calories and the 3 macronutrients (protein, fat, and carbohydrates), dietary content, or vitamins, including folic acid. The associations between maternal use of medications and supplements in early pregnancy and CHD in offspring are shown in Table 4. Anti‐rheumatic agents, antiepileptics other than valproic acid, and antimanic drugs were not used by anyone in the population with CHD. We observed positive significant associations between antihypertensive agents (OR, 3.90 [95% CI, 1.82–8.36]), valproic acid (OR, 3.77 [95% CI, 1.18–12.06]), and vitamin A supplementation (OR, 5.79 [95% CI, 2.32–14.42]) and offspring having or not having CHD. No other medications or supplements were significantly associated with CHD.
Table 2.
Association Between Background Characteristics of Participants and CHD in Offspring
| Characteristics | With CHD N=1264 | Without CHD N=90 400 | OR | (95% CI) | P value |
|---|---|---|---|---|---|
| Age at pregnancy, y | |||||
| <25 | 129 (10.2%) | 10 230 (11.3%) | … | … | … |
| 25–29 | 355 (28.1%) | 26 399 (29.2%) | 1.07 | (0.87–1.31) | 0.534 |
| 30–34 | 440 (34.8%) | 31 528 (34.9%) | 1.11 | (0.91–1.35) | 0.314 |
| 35–39 | 265 (21.0%) | 18 929 (20.9%) | 1.11 | (0.90–1.37) | 0.333 |
| ≥40 | 75 (6.0%) | 3295 (3.6%) | 1.81 | (1.35–2.41) | <0.001 |
| (Missing) | 0 (0.00%) | 19 (0.02%) | |||
| Maternal height, cm | |||||
| Mean±SD | 158.1±5.6 | 158.1±5.3 | 1.00 | (0.99–1.01) | 0.928 |
| (Missing) | 0 (0.00%) | 13 (0.01%) | |||
| Weight before pregnancy, kg | |||||
| Mean±SD | 53.4±9.5 | 53.1±8.8 | 1.00 | (1.00–1.01) | 0.291 |
| (Missing) | 1 (0.08%) | 29 (0.03%) | |||
| BMI before pregnancy, category | |||||
| 20–24 | 586 (46.4%) | 43 859 (48.5%) | … | … | |
| <20 | 520 (41.1%) | 36 923 (40.8%) | 1.05 | (0.94–1.19) | 0.385 |
| 25–29 | 117 (9.3%) | 7345 (8.1%) | 1.19 | (0.98–1.46) | 0.085 |
| ≥30 | 40 (3.2%) | 234 (2.5%) | 1.34 | (0.97–1.85) | 0.076 |
| (Missing) | 1 (0.08%) | 39 (0.04%) | |||
| Hemoglobin concentration, g/dL | |||||
| Mean±SD | 12.1±1.0 | 12.0±1.0 | 1.10 | (1.03–1.17) | 0.003 |
| (Missing) | 128 (10.1%) | 9883 (10.9%) | |||
| Infertility treatment | |||||
| No infertility treatment | 1124 (88.9%) | 81 926 (90.6%) | … | … | … |
| Infertility treatment other than in vitro fertilization | 83 (6.6%) | 5193 (5.7%) | 1.16 | (0.93–1.46) | 0.183 |
| In vitro fertilization | 54 (4.3%) | 2852 (3.2%) | 1.38 | (1.05–1.82) | 0.022 |
| (Missing) | 3 (0.2%) | 429 (0.47%) | |||
| Past maternal medical history | |||||
| CHD | 14 (1.1%) | 277 (0.31%) | 3.64 | (2.12–6.25) | <0.001 |
| Allergic disease | 711 (56.3%) | 50 161 (55.5%) | 1.03 | (0.92–1.15) | 0.588 |
| Immunologic disease | 14 (1.1%) | 600 (0.66%) | 1.68 | (0.98–2.86) | 0.057 |
| Neurologic/psychiatric disease | 122 (9.7%) | 7550 (8.4%) | 1.17 | (0.97–1.42) | 0.098 |
| Gastrointestinal disease | 2 (0.16%) | 241 (0.27%) | 0.59 | (0.15–2.39) | 0.462 |
| Diabetes | 42 (3.3%) | 2719 (3.0%) | 1.11 | (0.81–1.51) | 0.515 |
| Renal disease | 5 (0.40%) | 388 (0.43%) | 0.92 | (0.38–2.23) | 0.856 |
| Maternal education | |||||
| Junior high or high school graduate | 460 (36.4%) | 32 185 (35.6%) | … | … | … |
| Technical junior college or technical/vocational | 286 (22.6%) | 21 793 (24.1%) | 0.92 | (0.79–1.07) | 0.260 |
| College graduate or associate degree or higher | 491 (38.8%) | 35 067 (38.8%) | 0.98 | (0.86–1.11) | 0.753 |
| (Missing) | 27 (2.1%) | 1355 (1.5%) | |||
| Paternal education | |||||
| Junior high or high school graduate | 547 (43.3%) | 38 863 (43.0%) | … | … | … |
| Technical junior college or technical/vocational | 236 (18.7%) | 18 146 (20.1%) | 0.92 | (0.79–1.08) | 0.314 |
| College graduate or associate degree or higher | 440 (34.8%) | 31 466 (34.8%) | 0.99 | (0.88–1.13) | 0.919 |
| (Missing) | 41 (3.2%) | 1925 (2.1%) | |||
| Household income | |||||
| <2 million JPY | 65 (5.1%) | 4694 (5.2%) | … | … | … |
| 2 to <4 million JPY | 402 (31.8%) | 28 634 (31.7%) | 1.01 | (0.78–1.32) | 0.919 |
| 4 to <6 million JPY | 393 (31.1%) | 27 442 (30.4%) | 1.03 | (0.79–1.35) | 0.803 |
| 6 to <10 million JPY | 246 (19.5%) | 18 789 (20.8%) | 0.95 | (0.72–1.25) | 0.690 |
| ≥10 million JPY | 59 (4.7%) | 3549 (3.9%) | 1.20 | (0.84–1.71) | 0.313 |
| (Missing) | 99 (7.8%) | 7292 (8.1%) | |||
| Smoking around fertilization | 232 (18.4%) | 16 492 (18.2%) | 1.01 | (0.87–1.16) | 0.935 |
| (Missing) | 8 (0.63%) | 678 (0.75%) | |||
| Alcohol drinking around fertilization | 143 (11.3%) | 9056 (10.0%) | 1.14 | (0.96–1.36) | 0.136 |
| (Missing) | 3 (0.24%) | 425 (0.47%) | |||
| Maternal green tea intake, cups/wk, mean±SD | 6.3±11.8 | 5.7±10.4 | 1.00 | (1.00–1.01) | 0.058 |
| Maternal oolong tea intake, cups/wk, mean±SD | 2.4±7.5 | 2.3±7.3 | 1.00 | (0.99–1.01) | 0.745 |
| Maternal black tea intake, cups/wk, mean±SD | 1.3±3.1 | 1.4±3.5 | 0.99 | (0.97–1.01) | 0.326 |
| Maternal coffee intake, cups/wk, mean±SD | 3.3±7.0 | 3.2±6.6 | 1.00 | (0.99–1.01) | 0.821 |
Values in the table are n (%) unless otherwise noted. BMI indicates body mass index; CHD, congenital heart defects; JPY, Japanese Yen; and OR, odds ratio.
Table 3.
Crude Risk for Association Between Maternal Dietary Intake and CHD in Offspring
| Characteristic | With CHD N=1264 | Without CHD N=90 400 | OR | (95% CI) | P value |
|---|---|---|---|---|---|
| Total calories | |||||
| Q1: <1366 kcal/d | 314 (24.8%) | 22 599 (25.0%) | … | … | … |
| Q2: 1366–1690 kcal/d | 325 (25.7%) | 22 588 (25.0%) | 1.04 | (0.89–1.21) | 0.665 |
| Q3: 1691–2111 kcal/d | 310 (24.5%) | 22 603 (25.0%) | 0.99 | (0.84–1.16) | 0.904 |
| Q4: ≥2112 kcal/d | 314 (24.8%) | 22 598 (25.0%) | 1.00 | (0.85–1.17) | 0.982 |
| (Missing) | 1 (0.08%) | 12 (0.01%) | |||
| Total protein | |||||
| Q1: <44.0 g/d | 321 (25.4%) | 22 592 (25.0%) | … | … | … |
| Q2: 44.0–56.5 g/d | 295 (23.3%) | 22 618 (25.0%) | 0.92 | (0.78–1.08) | 0.306 |
| Q3: 56.6–73.2 g/d | 335 (26.5%) | 22 578 (25.0%) | 1.04 | (0.90–1.22) | 0.557 |
| Q4: ≥73.3 g/d | 312 (24.7%) | 22 600 (25.0%) | 0.97 | (0.83–1.14) | 0.726 |
| (Missing) | 1 (0.08%) | 12 (0.01%) | |||
| Total fat | |||||
| Q1: <40.6 g/d | 304 (24.1%) | 22 609 (25.0%) | … | … | … |
| Q2: 40.6–54.7 g/d | 325 (25.7%) | 22 588 (25.0%) | 1.07 | (0.91–1.25) | 0.392 |
| Q3: 54.8–73.6 g/d | 299 (23.7%) | 22 614 (25.0%) | 0.98 | (0.84–1.15) | 0.848 |
| Q4: ≥73.7 g/d | 335 (26.5%) | 22 577 (25.0%) | 1.10 | (0.94–1.29) | 0.225 |
| (Missing) | 1 (0.08%) | 12 (0.01%) | |||
| Total carbohydrate | |||||
| Q1: <189.8 g/d | 341 (27.0%) | 22 572 (25.0%) | … | … | … |
| Q2: 189.8–232.8 g/d | 310 (24.5%) | 22 603 (25.0%) | 0.91 | (0.78–1.06) | 0.234 |
| Q3: 232.9–285.7 g/d | 304 (24.1%) | 22 609 (25.0%) | 0.89 | (0.76–1.04) | 0.144 |
| Q4: ≥285.8 g/d | 308 (24.4%) | 22 604 (25.0%) | 0.90 | (0.77–1.05) | 0.190 |
| (Missing) | 1 (0.08%) | 12 (0.01%) | |||
| Total meat | |||||
| Q1: <38.99 g/d | 319 (25.2%) | 22 594 (25.0%) | … | … | … |
| Q2: 38.99–61.43 g/d | 317 (25.1%) | 22 596 (25.0%) | 0.99 | (0.85–1.16) | 0.234 |
| Q3: 61.44–93.23 g/d | 301 (23.8%) | 22 612 (25.0%) | 0.94 | (0.80–1.10) | 0.144 |
| Q4: ≥93.24 g/d | 326 (25.8%) | 22 586 (25.0%) | 1.02 | (0.88–1.19) | 0.190 |
| (Missing) | 1 (0.08%) | 12 (0.01%) | |||
| Total seafood | |||||
| Q1: <16.86 g/d | 320 (25.3%) | 22 593 (25.0%) | ‐ | ‐ | |
| Q2: 16.86–31.32 g/d | 288 (22.8%) | 22 625 (25.0%) | 0.90 | (0.77–1.05) | 0.221 |
| Q3: 31.33–50.47 g/d | 344 (27.2%) | 22 569 (25.0%) | 1.08 | (0.92–1.25) | 0.337 |
| Q4: ≥50.48 g/d | 311 (24.6%) | 22 601 (25.0%) | 0.97 | (0.83–1.14) | 0.725 |
| (Missing) | 1 (0.08%) | 12 (0.01%) | |||
| Total vegetables | |||||
| Q1: <96.42 g/d | 312 (24.7%) | 22 601 (25.0%) | … | … | … |
| Q2: 96.42–152.66 g/d | 315 (24.9%) | 22 598 (25.0%) | 1.01 | (0.86–1.18) | 0.943 |
| Q3: 152.7–231.8 g/d | 313 (24.8%) | 22 600 (25.0%) | 1.00 | (0.86–1.17) | 0.958 |
| Q4: ≥231.9 g/d | 323 (25.6%) | 22 589 (25.0%) | 1.04 | (0.89–1.21) | 0.717 |
| (Missing) | 1 (0.08%) | 12 (0.01%) | E | ||
| Green and yellow vegetables | |||||
| Q1: <42.83 g/d | 300 (23.7%) | 22 613 (25.0%) | … | … | … |
| Q2: 42.83–77.11 g/d | 324 (25.6%) | 22 589 (25.0%) | 1.08 | (0.92–1.27) | 0.318 |
| Q3: 77.12–129.66 g/d | 336 (26.6%) | 22 577 (25.0%) | 1.12 | (0.96–1.31) | 0.164 |
| Q4: ≥129.7 g/d | 303 (24.0%) | 22 609 (25.0%) | 1.01 | (0.86–1.19) | 0.867 |
| (Missing) | 1 (0.08%) | 12 (0.01%) | |||
| Total beans | |||||
| Q1: <18.07 g/d | 297 (23.5%) | 22 616 (25.0%) | … | … | … |
| Q2: 18.07–35.49 g/d | 330 (26.1%) | 22 583 (25.0%) | 1.11 | (0.95–1.30) | 0.187 |
| Q3: 35.50–68.92 g/d | 328 (25.9%) | 22 585 (25.0%) | 1.11 | (0.94–1.30) | 0.190 |
| Q4: ≥68.93 g/d | 308 (24.4%) | 22 604 (25.0%) | 1.04 | (0.88–1.22) | 0.618 |
| (Missing) | 1 (0.08%) | 12 (0.01%) | |||
| Retinol activity equivalent | |||||
| Q1: <262 μg/d | 302 (23.9%) | 22 611 (25.0%) | … | … | … |
| Q2: 262–405 μg/d | 316 (25.0%) | 22 597 (25.0%) | 1.05 | (0.89–1.23) | 0.566 |
| Q3: 406–633 μg/d | 324 (25.6%) | 22 589 (25.0%) | 1.07 | (0.92–1.26) | 0.378 |
| Q4: ≥634 μg/d | 321 (25.4%) | 22 591 (25.0%) | 1.06 | (0.91–1.25) | 0.426 |
| (Missing) | 1 (0.08%) | 12 (0.01%) | |||
| Vitamin C | |||||
| Q1: <53 mg/d | 311 (24.6%) | 22 602 (25.0%) | … | … | … |
| Q2: 53–80 mg/d | 309 (24.4%) | 22 604 (25.0%) | 0.99 | (0.85–1.16) | 0.967 |
| Q3: 81–119 mg/d | 320 (25.3%) | 22 593 (25.0%) | 1.03 | (0.88–1.20) | 0.702 |
| Q4: ≥120 mg/d | 323 (25.6%) | 22 589 (25.0%) | 1.04 | (0.89–1.22) | 0.620 |
| (Missing) | 1 (0.08%) | 12 (0.01%) | |||
| Vitamin K | |||||
| Q1: <107 μg/d | 326 (25.8%) | 22 587 (25.0%) | … | … | … |
| Q2: 107–161 μg/d | 301 (23.8%) | 22 612 (25.0%) | 0.92 | (0.79–1.08) | 0.288 |
| Q3: 162–249 μg/d | 315 (24.9%) | 22 598 (25.0%) | 0.97 | (0.83–1.13) | 0.650 |
| Q4: ≥250 μg/d | 321 (25.4%) | 22 591 (25.0%) | 0.98 | (0.84–1.15) | 0.880 |
| (Missing) | 1 (0.08%) | 12 (0.01%) | |||
| Folic acid | |||||
| Q1: <178 μg/d | 296 (23.4%) | 22 617 (25.0%) | … | … | … |
| Q2: 178–246 μg/d | 337 (26.7%) | 22 576 (25.0%) | 1.14 | (0.97–1.33) | 0.103 |
| Q3: 247–338 μg/d | 287 (22.7%) | 22 626 (25.0%) | 0.97 | (0.82–1.14) | 0.696 |
| Q4: ≥339 μg/d | 343 (27.1%) | 22 569 (25.0%) | 1.16 | (0.99–1.36) | 0.061 |
| (Missing) | 1 (0.08%) | 12 (0.01%) | |||
Values in the table are n (%) unless otherwise noted. CHD indicates congenital heart defects; OR, odds ratio; and Q, quartile.
Table 4.
Crude Risk for Association Between Maternal Medication/Supplementation Use and CHD in Offspring
| Medication/supplement | With CHD N=1264 | Without CHD N=90 400 | OR | (95% CI) | P value |
|---|---|---|---|---|---|
| Corticosteroid | 28 (2.2%) | 2696 (3.0%) | 0.74 | (0.51–1.07) | 0.112 |
| Anti‐rheumatic agent | 0 (0.00%) | 42 (0.05%) | Inf | ||
| Insulin | 3 (0.24%) | 133 (0.15%) | 1.61 | (0.51–5.08) | 0.412 |
| Hypoglycemic agent | 1 (0.08%) | 96 (0.11%) | 0.74 | (0.10–5.35) | 0.770 |
| Antihyperlipidemic agent | 1 (0.08%) | 24 (0.03%) | 2.98 | (0.40–22.06) | 0.285 |
| Hormonal agent | 23 (1.8%) | 1689 (1.9%) | 0.97 | (0.64–1.47) | 0.899 |
| Antihypertensive agent | 7 (0.55%) | 129 (0.14%) | 3.90 | (1.82–8.36) | <0.001 |
| Psychotropic agent | |||||
| SSRI | 3 (0.24%) | 155 (0.17%) | 1.39 | (0.44–4.35) | 0.577 |
| Antidepressant other than SSRI | 2 (0.16%) | 79 (0.09%) | 1.81 | (0.44–7.38) | 0.407 |
| Anti‐anxiety agent | 6 (0.47%) | 315 (0.35%) | 1.36 | (0.61–3.07) | 0.452 |
| Narcotic sleep agent | 4 (0.32%) | 215 (0.24%) | 1.33 | (0.49–3.59) | 0.571 |
| Valproic acid | 3 (0.24%) | 57 (0.06%) | 3.77 | (1.18–12.06) | 0.025 |
| Antiepileptic agent other than valproic acid | 0 (0.00%) | 119 (0.13%) | Inf | ||
| Antimanic agent | 0 (0.00%) | 3 (0.00%) | Inf | ||
| Supplements | |||||
| Vitamin A | 5 (0.40%) | 62 (0.07%) | 5.79 | (2.32–14.42) | <0.001 |
| Vitamin B | 28 (2.2%) | 2209 (2.4%) | 0.90 | (0.62–1.32) | 0.601 |
| Vitamin C | 14 (1.1%) | 1567 (1.7%) | 0.63 | (0.37–1.08) | 0.092 |
| Vitamin D | 2 (0.16%) | 121 (0.13%) | 1.18 | (0.29–4.79) | 0.814 |
| Vitamin E | 7 (0.55%) | 313 (0.35%) | 1.60 | (0.76–3.40) | 0.218 |
| Folic acid | 338 (26.7%) | 24 912 (27.6%) | 0.96 | (0.85–1.09) | 0.519 |
| Multivitamin | 37 (2.9%) | 2674 (3.0%) | 0.99 | (0.71–1.38) | 0.949 |
| Comprehensive supplement | 31 (2.5%) | 2325 (2.6%) | 0.95 | (0.67–1.36) | 0.790 |
Values in the table are n (%) unless otherwise noted. CHD indicates congenital heart defects; Inf, infimum; OR, odds ratio; and SSRI, selective serotonin reuptake inhibitor.
Multivariable Analysis
All exposure factors that were significantly associated in the univariable analysis and other factors that have been suggested to be associated with CHD in previous reports, including body mass index, maternal history of diabetes, smoking, alcohol consumption, and dietary intake of retinol and folic acid, were evaluated as explanatory variables in the multivariable logistic regression model (Table 5). Age ≥40 years at pregnancy (aOR, 1.59 [95% CI, 1.14–2.20]); past maternal history of CHD (aOR, 3.42 [95% CI, 1.91–6.13]); hemoglobin concentration (aOR, 1.10 [95% CI, 1.03–1.17]); and maternal use of valproic acid (aOR, 4.85 [95% CI, 1.51–15.64]), vitamin A supplements (aOR, 5.78 [95% CI, 2.30–14.51]), and antihypertensive agents (aOR, 3.80 [95% CI, 1.74–8.29]) were significantly associated with CHD in the multivariable model. However, infertility treatment showed no significant association in the multivariable analysis.
Table 5.
Multivariable Analysis Exploring Association Between Maternal Medication/Supplement Use During Early Pregnancy and CHD in Offspring
| Adjusted OR | (95% CI) | P value | |
|---|---|---|---|
| Age at pregnancy (reference: <25 y) | |||
| 25–29 y | 1.06 | (0.85–1.33) | 0.592 |
| 30–34 y | 1.10 | (0.88–1.37) | 0.396 |
| 35–39 y | 1.08 | (0.85–1.37) | 0.511 |
| ≥40 y | 1.59 | (1.14–2.20) | 0.006 |
| BMI at pregnancy (reference: 20–24 kg/m2) | |||
| <20 kg/m2 | 1.07 | (0.94–1.21) | 0.320 |
| 25–29 kg/m2 | 1.11 | (0.90–1.38) | 0.319 |
| ≥30 kg/m2 | 1.20 | (0.85–1.70) | 0.307 |
| Past maternal history of CHD | 3.42 | (1.91–6.13) | <0.001 |
| Past maternal history of diabetes | 1.11 | (0.81–1.53) | 0.513 |
| Hemoglobin concentration | 1.10 | (1.03–1.17) | 0.003 |
| Smoking around fertilization | 1.03 | (0.88–1.20) | 0.753 |
| Valproic acid | 4.86 | (1.51–15.64) | 0.008 |
| Dietary intake of retinol activity equivalent (reference: Q1: <262 μg/d) | |||
| Q2: 262–405 μg/d | 1.03 | (0.86–1.24) | 0.749 |
| Q3: 406–633 μg/d | 1.06 | (0.86–1.30) | 0.591 |
| Q4: ≥634 μg/d | 0.97 | (0.77–1.22) | 0.823 |
| Dietary intake of folic acid (reference: Q1: <178 μg/d) | |||
| Q2: 178–246 μg/d | 1.08 | (0.90–1.30) | 0.440 |
| Q3: 247–338 μg/d | 0.90 | (0.73–1.11) | 0.345 |
| Q4: ≥339 μg/d | 1.13 | (0.90–1.43) | 0.305 |
| Alcohol drinking after fertilization | 1.07 | (0.89–1.30) | 0.461 |
| Vitamin A supplementation | 5.78 | (2.30–14.51) | <0.001 |
| Infertility treatment (reference: no infertility treatment) | |||
| Infertility treatment other than in vitro fertilization | 1.19 | (0.94–1.50) | 0.153 |
| In vitro fertilization | 1.25 | (0.92–1.68) | 0.151 |
| Antihypertensive agent use | 3.80 | (1.74–8.29) | <0.001 |
BMI indicates body mass index; CHD, congenital heart defects; OR, odds ratio; and Q, quartile.
Sensitivity Analysis
The results of sensitivity analyses, which excluded offspring with trisomy 21, 18, and 13, are shown in Tables S1 through S5. Similarly, past maternal history of CHD (aOR, 3.15 [95% CI, 1.67–5.95]); hemoglobin concentration (aOR, 1.07 [95% CI, 1.01–1.15]); and use of valproic acid (aOR, 5.15 [95% CI, 1.60–16.58]), vitamin A supplements (aOR, 5.13 [95% CI, 1.85–14.21]), and antihypertensive agents (aOR, 3.19 [95% CI, 1.28–7.92]) were significantly associated with CHD in offspring in the multivariable model; age at pregnancy did not show a significant association with CHD in offspring (Table S5).
DISCUSSION
This secondary analysis of a large‐scale prospective birth cohort study revealed that, among the maternal factors during pregnancy comprehensively examined, age at pregnancy, past history of maternal CHD, higher maternal hemoglobin concentration, maternal use of valproic acid or antihypertensive medicine, and vitamin A supplementation were significant risk factors associated with CHD in offspring. However, parental socioeconomic status, maternal dietary intake, and maternal lifestyle habits did not show a significant association with CHD in offspring.
We found that maternal intake of valproic acid, antihypertensive medication, and vitamin A during early pregnancy was associated with a 3‐ to 6‐fold increased risk of CHD in offspring. In a systematic review and meta‐analysis that combined 2 cohort studies (n=502), Weston et al 23 reported that maternal intake of valproic acid carries a 16‐fold increased risk of CHD in offspring in comparison with healthy pregnant women. Similarly, a systematic review and meta‐analysis by Ramakrishnan et al compared the risk of CHD in treated and untreated maternal hypertension with that of healthy pregnant women. 24 Those authors reported that mothers treated for hypertension have twice the risk of CHD as mothers without hypertension, compared with a 1.4 times greater risk in mothers with untreated hypertension. Although the risk estimates were not entirely consistent with previous reports, the results of our study support the need to carefully balance the risks and benefits of valproic acid or antihypertensive therapy in pregnant women and women of childbearing age and to consider switching to other drugs if clinically feasible. Retinoic acid, which is a vitamin A metabolite, acts as a teratogen, and excess or defective retinoic acid signaling causes congenital defects, including CHD. 25 Two epidemiological studies have suggested that maternal vitamin A supplementation during the first 12 weeks of gestation, 4 or even in the year before pregnancy, increased the risk of CHD, 26 resulting in a >4‐fold increased risk of conotruncal heart defects; however, these studies have not been replicated by other investigators. 27 The results of our study supported the theory that vitamin A supplementation in early pregnancy increases the risk of developing CHD. Additionally, maternal dietary intake of vitamin A was <3000 μg retinol activity equivalent/d in all participants, which is considered the threshold for the development of CHD. Therefore, CHD occurred as a result of maternal vitamin A supplementation rather than excessive maternal dietary vitamin A intake. Women who are pregnant or planning to become pregnant should be advised not to take vitamin A supplements to avoid increasing the risk of CHD in their offspring.
Maternal history of CHD, age >40 years at pregnancy, and hemoglobin concentration were significant risk factors associated with CHD in the present study. It is known that ≈15% of all CHD cases have a genetic cause (eg, 8%–10% have aneuploidy and 3%–5% have single‐gene defects). 2 , 28 Epidemiological studies have reported that offspring born to mothers with CHD have a 2‐ to 10‐times greater risk of recurrent CHD than those born to mothers without CHD, which is consistent with our study findings. 29 , 30 , 31 Offspring born to pregnant women >40 years of age have a higher proportion of chromosomal abnormalities such as trisomy 18 and 21, and offspring with trisomies have a >50% probability of CHD. 32 , 33 In the sensitivity analysis excluding offspring with trisomy 21, 18, and 13, age at pregnancy did not show statistical significance in the multivariable model (Table S5). Thus, the association between age and CHD in this study is likely to be confounded by the presence of other genetic abnormalities.
In this study, higher maternal hemoglobin levels were associated with CHD in offspring. However, 2 large population‐based cohort studies 10 , 11 have reported that mothers with a history of anemia or anemia‐related disease are more likely to have offspring with CHD. Furthermore, several animal studies have shown that maternal hypoxia induces the development of fetal CHD 34 , 35 ; the results of the present study seemingly contradict these previous reports. However, in the present study, the time of examination was during the second trimester, which may not reflect hemoglobin concentrations during the first trimester, the period of organogenesis. Furthermore, the hemoglobin concentrations among pregnant women with and without CHD in offspring were nearly identical (absolute mean difference 0.1 mg/dL). We do not know why this subtle difference was observed as a putative risk of CHD in our study, and it is not a clinically significant difference to be diagnosed as anemia or polycythemia; therefore, we might observe a spurious association caused by unknown confounding factors. We also believe that this clinically unsignificant difference in maternal hemoglobin does not rule out the previously reported association between maternal anemia and CHD in offspring. Further studies are needed to confirm the association between high maternal hemoglobin and CHD in offspring.
Several maternal factors that have been previously reported to be associated with CHD in offspring, such as maternal diabetes, maternal alcohol intake, and smoking, were not found to be significantly associated in the present study. Maternal diabetes has been reported to be a main cause of cardiac teratogenicity owing to excessive glucose supply to the fetus, 36 with a 2‐ to 5‐fold increased risk of CHD in offspring. 37 , 38 However, gestational diabetes has been reported to be associated with a lower risk of CHD than maternal diabetes. 38 , 39 In this study, only 5% of pregnant women categorized with diabetes used insulin or other diabetes medication; therefore, most cases of diabetes would be gestational diabetes. We speculate that the high proportion of gestational diabetes in this cohort may be the reason why we were unable to detect an association between maternal diabetes and CHD. Regarding maternal smoking, a recent systematic review and meta‐analysis by Zhao et al 40 reported a weak association between maternal smoking and CHD, with a combined risk ratio <1.3. Similarly, in a systematic review and meta‐analysis, Sun et al 41 reported no significant association between maternal alcohol consumption and risk of CHD. In Japan, all pregnant women are instructed to stop smoking and drinking alcohol during pregnancy. Therefore, it is assumed that exposure to smoking and alcohol consumption were not sufficient to test their effects in this study.
Folic acid is an essential vitamin for purine and thymidine synthesis and as a methyl donor for DNA methylation. Maternal folic acid supplementation prevents the development of neural tube defects. 42 However, its effectiveness in preventing CHD among offspring remains controversial. 16 , 43 , 44 Xu et al 45 conducted a meta‐analysis showing that maternal folic acid supplementation was significantly associated with a decreased risk of CHDs based on 20 case–control studies; however, significant heterogeneity existed among studies from different areas (North America, Europe, or China), and its effect size differed in each area. The effect of folic acid supplements in preventing CHD possibly varies among regions because of differences in dietary intake and lifestyle.
The present study had several limitations. First, the study design was observational; therefore, there could be unmeasured confounding factors that we could not adjust. Information on exposures in the first trimester—the period of organogenesis—may not have been accurately obtained because we recruited most pregnant women during the second trimester in most of them. Second, this study included information obtained using a self‐report questionnaire. Self‐reporting may lead to decreased measurement accuracy compared with direct measurement and data collection by medical staff. Therefore, we should consider the possibility of recall bias. In addition, details of diseases such as hypertension were not reported. Third, we were unable to investigate the association between paternal past history of CHD and CHD in offspring because of missing data. Similarly, it is important to be aware of the potential for bias owing to loss to follow‐up. Fourth, the first trimester is the period when the fetal heart is forming and congenital heart disease occurs: however, we measured maternal environmental or clinical factors during the second trimester. Finally, genetic abnormalities contribute substantially to the development of CHD; however, in this study, we only investigated the presence or absence of trisomy 21, 18, and 13. Because the questionnaire we used for this study did not include any questions related to microarray analysis, no sensitivity analysis was performed to exclude patients with genetic abnormalities such as deletion 22q11.2. Thus, there is a possibility of significant bias.
In conclusion, we revealed that maternal factors, including age at pregnancy; past history of CHD; higher hemoglobin concentration; and use of valproic acid, antihypertensive medication, and vitamin A, were associated with CHD in offspring. To reduce the risk of CHD in offspring, education in avoiding teratogenic medicines and supplements for women who wish to become pregnant is important.
APPENDIX
Members of the JECS Group as of 2022
Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan), Shin Yamazakii (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Tomotaka Sobue (Osaka University, Suita, Japan), Masayuki Shima (Hyogo Medical University, Nishinomiya, Japan), Hiroshige Nakamura (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan), Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
Sources of Funding
This study was funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the above government.
Disclosures
None.
Supporting information
Data S1
Tables S1–S5
Acknowledgments
The authors would like to express our gratitude to everyone who participated in this study and to all those who were involved in the data collection. We thank Analisa Avila, MPH, ELS, of Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript.
This article was sent to Mahasin S. Mujahid, PhD, MS, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.029268
For Sources of Funding and Disclosures, see page 12.
References
- 1. Wu W, He J, Shao X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990–2017. Medicine (Baltimore). 2020;99:e20593. doi: 10.1097/MD.0000000000020593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morton SU, Quiat D, Seidman JG, Seidman CE. Genomic frontiers in congenital heart disease. Nat Rev Cardiol. 2022;19:26–42. doi: 10.1038/s41569-021-00587-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smithells RW, Newman CG. Recognition of thalidomide defects. J Med Genet. 1992;29:716–723. doi: 10.1136/jmg.29.10.716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rothman KJ, Moore LL, Singer MR, Nguyen US, Mannino S, Milunsky A. Teratogenicity of high vitamin a intake. N Engl J Med. 1995;333:1369–1373. doi: 10.1056/nejm199511233332101 [DOI] [PubMed] [Google Scholar]
- 5. Campbell E, Kennedy F, Russell A, Smithson WH, Parsons L, Morrison PJ, Liggan B, Irwin B, Delanty N, Hunt SJ, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland epilepsy and pregnancy registers. J Neurol Neurosurg Psychiatry. 2014;85:1029–1034. doi: 10.1136/jnnp-2013-306318 [DOI] [PubMed] [Google Scholar]
- 6. Patorno E, Huybrechts KF, Bateman BT, Cohen JM, Desai RJ, Mogun H, Cohen LS, Hernandez‐Diaz S. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376:2245–2254. doi: 10.1056/NEJMoa1612222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Csáky‐Szunyogh M, Vereczkey A, Gerencsér B, Czeizel AE. Maternal hypertension with nifedipine treatment associated with a higher risk for right‐sided obstructive defects of the heart: a population‐based case‐control study. Heart Asia. 2014;6:3–7. doi: 10.1136/heartasia-2013-010331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooper WO, Hernandez‐Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, Hall K, Ray WA. Major congenital malformations after first‐trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443–2451. doi: 10.1056/NEJMoa055202 [DOI] [PubMed] [Google Scholar]
- 9. Tikkanen J, Heinonen OP. Risk factors for cardiovascular malformations in Finland. Eur J Epidemiol. 1990;6:348–356. doi: 10.1007/bf00151707 [DOI] [PubMed] [Google Scholar]
- 10. Chou HH, Chiou MJ, Liang FW, Chen LH, Lu TH, Li CY. Association of maternal chronic disease with risk of congenital heart disease in offspring. Can Med Assoc J. 2016;188:e438–e446. doi: 10.1503/cmaj.160061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu S, Joseph KS, Lisonkova S, Rouleau J, Van den Hof M, Sauve R, Kramer MS. Association between maternal chronic conditions and congenital heart defects: a population‐based cohort study. Circulation. 2013;128:583–589. doi: 10.1161/circulationaha.112.001054 [DOI] [PubMed] [Google Scholar]
- 12. Kurita H, Motoki N, Inaba Y, Misawa Y, Ohira S, Kanai M, Tsukahara T, Nomiyama T; the Japan Environment & Children's Study (JECS) Group . Maternal alcohol consumption and risk of offspring with congenital malformation: the Japan environment and Children's study. Pediatr Res. 2021;90:479–486. doi: 10.1038/s41390-020-01274-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Motoki N, Inaba Y, Shibazaki T, Misawa Y, Ohira S, Kanai M, Kurita H, Nakazawa Y, Tsukahara T, Nomiyama T, et al. Maternal exposure to housing renovation during pregnancy and risk of offspring with congenital malformation: the Japan environment and Children's study. Sci Rep. 2019;9:11564. doi: 10.1038/s41598-019-47925-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ito K, Hanaoka T, Tamura N, Sasaki S, Miyashita C, Araki A, Ito S, Minakami H, Cho K, Endo T, et al. Association between maternal serum folate concentrations in the first trimester and the risk of birth defects: the Hokkaido study of environment and Children's health. J Epidemiol. 2019;29:164–171. doi: 10.2188/jea.JE20170185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dale MTG, Magnus P, Leirgul E, Holmstrom H, Gjessing HK, Brodwall K, Haugen M, Stoltenberg C, Oyen N. Intake of sucrose‐sweetened soft beverages during pregnancy and risk of congenital heart defects (CHD) in offspring: a Norwegian pregnancy cohort study. Eur J Epidemiol. 2019;34:383–396. doi: 10.1007/s10654-019-00480-y [DOI] [PubMed] [Google Scholar]
- 16. Oyen N, Olsen SF, Basit S, Leirgul E, Strom M, Carstensen L, Granstrom C, Tell GS, Magnus P, Vollset SE, et al. Association between maternal folic acid supplementation and congenital heart defects in offspring in birth cohorts from Denmark and Norway. J Am Heart Assoc. 2019;8:e011615. doi: 10.1161/JAHA.118.011615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hjortebjerg D, Andersen AM, Garne E, Raaschou‐Nielsen O, Sørensen M. Non‐occupational exposure to paint fumes during pregnancy and risk of congenital anomalies: a cohort study. Environ Health. 2012;11:54. doi: 10.1186/1476-069x-11-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pedersen M, Garne E, Hansen‐Nord N, Hjortebjerg D, Ketzel M, Raaschou‐Nielsen O, Nybo Andersen AM, Sorensen M. Exposure to air pollution and noise from road traffic and risk of congenital anomalies in the Danish National Birth Cohort. Envir Res. 2017;159:39–45. doi: 10.1016/j.envres.2017.07.031 [DOI] [PubMed] [Google Scholar]
- 19. Schmidt AB, Lund M, Corn G, Halldorsson TI, Oyen N, Wohlfahrt J, Olsen SF, Melbye M. Dietary glycemic index and glycemic load during pregnancy and offspring risk of congenital heart defects: a prospective cohort study. Am J Clin Nutr. 2020;111:526–535. doi: 10.1093/ajcn/nqz342 [DOI] [PubMed] [Google Scholar]
- 20. Strandberg‐Larsen K, Skov‐Ettrup LS, Gronbaek M, Andersen AM, Olsen J, Tolstrup J. Maternal alcohol drinking pattern during pregnancy and the risk for an offspring with an isolated congenital heart defect and in particular a ventricular septal defect or an atrial septal defect. Birth Defects Res Part A: Clin Mol Teratol. 2011;91:616–622. doi: 10.1002/bdra.20818 [DOI] [PubMed] [Google Scholar]
- 21. Kawamoto T, Nitta H, Murata K, Toda E, Tsukamoto N, Hasegawa M, Yamagata Z, Kayama F, Kishi R, Ohya Y, et al. Rationale and study design of the Japan environment and children's study (JECS). BMC Public Health. 2014;14:25. doi: 10.1186/1471-2458-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yokoyama Y, Takachi R, Ishihara J, Ishii Y, Sasazuki S, Sawada N, Shinozawa Y, Tanaka J, Kato E, Kitamura K, et al. Validity of short and long self‐administered food frequency questionnaires in ranking dietary intake in middle‐aged and elderly Japanese in the Japan public health center‐based prospective study for the Next generation (JPHC‐NEXT) protocol area. J Epidemiol. 2016;26:420–432. doi: 10.2188/jea.JE20150064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weston J, Bromley R, Jackson CF, Adab N, Clayton‐Smith J, Greenhalgh J, Hounsome J, McKay AJ, Tudur Smith C, Marson AG. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database Syst Rev. 2016;11:CD010224. doi: 10.1002/14651858.CD010224.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramakrishnan A, Lee LJ, Mitchell LE, Agopian AJ. Maternal hypertension during pregnancy and the risk of congenital heart defects in offspring: a systematic review and meta‐analysis. Pediatr Cardiol. 2015;36:1442–1451. doi: 10.1007/s00246-015-1182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakajima Y. Retinoic acid signaling in heart development. Genesis. 2019;57:e23300. doi: 10.1002/dvg.23300 [DOI] [PubMed] [Google Scholar]
- 26. Botto LD, Loffredo C, Scanlon KS, Ferencz C, Khoury MJ, David Wilson P, Correa A. Vitamin A and cardiac outflow tract defects. Epidemiology. 2001;12:491–496. doi: 10.1097/00001648-200109000-00005 [DOI] [PubMed] [Google Scholar]
- 27. Mills JL, Simpson JL, Cunningham GC, Conley MR, Rhoads GG. Vitamin A and birth defects. Am J Obstet Gynecol. 1997;177:31–36. doi: 10.1016/S0002-9378(97)70434-4 [DOI] [PubMed] [Google Scholar]
- 28. Kodo K, Uchida K, Yamagishi H. Genetic and cellular interaction during cardiovascular development implicated in congenital heart diseases. Front Cardiovasc Med. 2021;8:653244. doi: 10.3389/fcvm.2021.653244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burn J, Brennan P, Little J, Holloway S, Coffey R, Somerville J, Dennis NR, Allan L, Arnold R, Deanfield JE, et al. Recurrence risks in offspring of adults with major heart defects: results from first cohort of British collaborative study. Lancet. 1998;351:311–316. doi: 10.1016/s0140-6736(97)06486-6 [DOI] [PubMed] [Google Scholar]
- 30. Yokouchi‐Konishi T, Yoshimatsu J, Sawada M, Shionoiri T, Nakanishi A, Horiuchi C, Tsuritani M, Iwanaga N, Kamiya CA, Neki R, et al. Recurrent congenital heart diseases among neonates born to mothers with congenital heart diseases. Pediatr Cardiol. 2019;40:865–870. doi: 10.1007/s00246-019-02083-6 [DOI] [PubMed] [Google Scholar]
- 31. Takatsuki S, Furutani Y, Inai K, Kobayashi T, Inuzuka R, Uyeda T, Kamisago M, Muneuchi J, Kaneko M, Misaki Y, et al. Pregnancy and delivery in patients with repaired congenital heart disease—a retrospective Japanese multicenter study. Circ J. 2020;84:2270–2274. doi: 10.1253/circj.CJ-19-1150 [DOI] [PubMed] [Google Scholar]
- 32. Cuckle H, Morris J. Maternal age in the epidemiology of common autosomal trisomies. Prenat Diagn. 2021;41:573–583. doi: 10.1002/pd.5840 [DOI] [PubMed] [Google Scholar]
- 33. Pierpont ME, Brueckner M, Chung WK, Garg V, Lacro RV, McGuire AL, Mital S, Priest JR, Pu WT, Roberts A, et al. Genetic basis for congenital heart disease: revisited: a scientific statement from the American Heart Association. Circulation. 2018;138:e653–e711. doi: 10.1161/cir.0000000000000606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shi H, O'Reilly VC, Moreau JL, Bewes TR, Yam MX, Chapman BE, Grieve SM, Stocker R, Graham RM, Chapman G, et al. Gestational stress induces the unfolded protein response, resulting in heart defects. Development. 2016;143:2561–2572. doi: 10.1242/dev.136820 [DOI] [PubMed] [Google Scholar]
- 35. Moumne O, Chowdhurry R, Doll C, Pereira N, Hashimi M, Grindrod T, Dollar JJ, Riva A, Kasahara H. Mechanism sharing between genetic and gestational hypoxia‐induced cardiac anomalies. Front Cardiovasc Med. 2018;5:100. doi: 10.3389/fcvm.2018.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakano H, Fajardo VM, Nakano A. The role of glucose in physiological and pathological heart formation. Dev Biol. 2021;475:222–233. doi: 10.1016/j.ydbio.2021.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Øyen N, Diaz LJ, Leirgul E, Boyd HA, Priest J, Mathiesen ER, Quertermous T, Wohlfahrt J, Melbye M. Prepregnancy diabetes and offspring risk of congenital heart disease: a nationwide cohort study. Circulation. 2016;133:2243–2253. doi: 10.1161/circulationaha.115.017465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu Y, Liu B, Sun Y, Du Y, Santillan MK, Santillan DA, Snetselaar LG, Bao W. Association of maternal prepregnancy diabetes and gestational diabetes mellitus with congenital anomalies of the newborn. Diabetes Care. 2020;43:2983–2990. doi: 10.2337/dc20-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Papazoglou AS, Moysidis DV, Panagopoulos P, Kaklamanos EG, Tsagkaris C, Vouloagkas I, Karagiannidis E, Tagarakis GI, Papamitsou T, Papanikolaou IG, et al. Maternal diabetes mellitus and its impact on the risk of delivering a child with congenital heart disease: a systematic review and meta‐analysis. J Matern‐Fetal Neonatal Med. 2021;35:7685–7694. doi: 10.1080/14767058.2021.1960968 [DOI] [PubMed] [Google Scholar]
- 40. Zhao L, Chen L, Yang T, Wang L, Wang T, Zhang S, Chen L, Ye Z, Zheng Z, Qin J. Parental smoking and the risk of congenital heart defects in offspring: an updated meta‐analysis of observational studies. Eur J Prev Cardiol. 2020;27:1284–1293. doi: 10.1177/2047487319831367 [DOI] [PubMed] [Google Scholar]
- 41. Sun J, Chen X, Chen H, Ma Z, Zhou J. Maternal alcohol consumption before and during pregnancy and the risks of congenital heart defects in offspring: a systematic review and meta‐analysis. Congenit Heart Dis. 2015;10:E216–E224. doi: 10.1111/chd.12271 [DOI] [PubMed] [Google Scholar]
- 42. van Gool JD, Hirche H, Lax H, De Schaepdrijver L. Folic acid and primary prevention of neural tube defects: a review. Reprod Toxicol. 2018;80:73–84. doi: 10.1016/j.reprotox.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 43. Mao B, Qiu J, Zhao N, Shao Y, Dai W, He X, Cui H, Lin X, Lv L, Tang Z, et al. Maternal folic acid supplementation and dietary folate intake and congenital heart defects. PLoS One. 2017;12:e0187996. doi: 10.1371/journal.pone.0187996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Feng Y, Cai J, Tong X, Chen R, Zhu Y, Xu B, Mo X. Non‐inheritable risk factors during pregnancy for congenital heart defects in offspring: a matched case‐control study. Int J Cardiol. 2018;264:45–52. doi: 10.1016/j.ijcard.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 45. Xu A, Cao X, Lu Y, Li H, Zhu Q, Chen X, Jiang H, Li X. A meta‐analysis of the relationship between maternal folic acid supplementation and the risk of congenital heart defects. Int Heart J. 2016;57:725–728. doi: 10.1536/ihj.16-054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S5


