Abstract
Background
The association between psychosocial factors and atrial fibrillation (AF) is poorly understood.
Methods and Results
Postmenopausal women from the Women's Health Initiative were retrospectively analyzed to identify incident AF in relation to a panel of validated psychosocial exposure variables, as assessed by multivariable Cox proportional hazard regression and hierarchical cluster analysis. Among the 83 736 women included, the average age was 63.9±7.0 years. Over an average of 10.5±6.2 years follow‐up, there were 23 954 cases of incident AF. Hierarchical cluster analysis generated 2 clusters of highly correlated psychosocial variables: the Stress Cluster included stressful life events, depressive symptoms, and insomnia, and the Strain Cluster included optimism, social support, social strain, cynical hostility, and emotional expressiveness. Incident AF was associated with higher values in the Stress Cluster (hazard ratio [HR], 1.07 per unit cluster score [95% CI, 1.05–1.09]) and the Strain Cluster (HR, 1.03 per unit cluster score [95% CI, 1.00–1.05]). Of the 8 individual psychosocial predictors that were tested, insomnia (HR, 1.04 [95% CI, 1.03–1.06]) and stressful life events (HR, 1.02 [95% CI, 1.01–1.04]) were most strongly associated with increased incidence of AF in Cox regression analysis after multivariate adjustment. Subgroup analyses showed that the Strain Cluster was more strongly associated with incident AF in those with lower traditional AF risks (P for interaction=0.02) as determined by the cohorts for heart and aging research in genomic epidemiology for atrial fibrillation score.
Conclusions
Among postmenopausal women, 2 clusters of psychosocial stressors were found to be significantly associated with incident AF. Further research is needed to validate these associations.
Keywords: atrial fibrillation, hierarchical cluster analysis, psychosocial clusters, strain, stress, women's health
Subject Categories: Cardiovascular Disease, Mental Health, Risk Factors, Women
Nonstandard Abbreviations and Acronyms
- HCA
hierarchical cluster analysis
- SLE
stressful life events
- WHI
Women's Health Initiative
Clinical Perspective.
What Is New?
Emerging studies show that psychosocial factors can potentially impact atrial fibrillation (AF) via various mechanisms, although data are limited to a few individual stressors, and information in older women remains limited.
In this study, we used hierarchical cluster analysis to analyze the association between a panel of 8 psychosocial stressors and incident AF in 83 736 postmenopausal women in the Women's Health Initiative studies.
Two distinct clusters, the Stress Cluster (including stressful life events, depressive symptoms, and insomnia) and the Strain Cluster (including optimism, social support, social strain, cynical hostility, and emotional expressiveness) were found to be significantly associated with AF incidence after adjusting for traditional risk factors.
What Are the Clinical Implications?
Established AF risk factors such as older age and atherometabolic diseases do not fully explain AF risk, and it is important to explore novel determinants of AF in older women, because they generally live longer and are more prone to develop adverse outcomes as a result of AF.
The grouping of psychosocial stressors into the Stress and Strain Clusters from this study presents a comprehensive appraisal of the heart–brain interactions in the development of AF in postmenopausal women.
Further prospective investigations are needed to confirm these associations and to evaluate whether customized stress‐relieving interventions based on each individual's Stress/Strain Cluster profile may modify AF risk.
Atrial fibrillation (AF) is the most common cardiac arrhythmia with high prevalence, morbidity, mortality, and economic burden. 1 , 2 , 3 Some studies suggest sex‐specific differences in AF pathophysiology between men and women, and an association of AF with worse outcomes among women. 4 , 5 , 6 In addition to the traditional AF risk factors such as advanced age, hypertension, diabetes, coronary artery disease, heart failure, and obesity, 7 and emotional and psychological distress from stress, anxiety, insomnia, and depressive symptoms potentially impact AF by activating inflammation and neurohormonal pathways. 8 , 9 , 10 How these highly prevalent psychosocial factors may affect AF in postmenopausal women remains poorly understood with conflicting reports in the literature. 11 , 12 , 13
With its large cohort size, sizeable incident case numbers, long follow‐up period, and detailed psycho–social–behavioral documentation at baseline, the Women's Health Initiative (WHI) offers an ideal platform for examining the relationship between psychosocial risks and AF. 14 , 15 We hypothesized that insomnia and other psychosocial characteristics or clusters of these factors with shared conceptual similarities would be associated with incident AF in eligible postmenopausal women from the WHI clinical trials and observational study. We further hypothesized that psychosocial predictors may have a stronger association with AF incidence in participants with a lower prevalence of traditional AF risk factors.
Methods
Data Availability Statement
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the WHI at email: p&p@whi.org or website: https://www.whi.org/propose‐a‐paper.
Study Population and Design
This study included all eligible participants between the ages of 50 and 79 years recruited at US clinical centers between 1994 and 1998 who enrolled in the WHI randomized controlled trials and observational study. At enrollment in the WHI, participants completed baseline questionnaires detailing their demographics, medical history, and health habits, and underwent baseline vital signs measurement and laboratory testing. Extensive descriptions of the study design, inclusion criteria, data collection, validation, and monitoring have been thoroughly documented previously. 16 , 17 , 18 , 19
We excluded participants with AF at baseline as reported on the initial questionnaire completed by all subjects or who had AF identified on baseline ECG. We also excluded participants who were never enrolled in Fee‐for‐Service (FFS) A+B of the Centers for Medicare or Medicaid Services, or who had missing data on covariates.
The WHI studies were reviewed and approved by the institutional review boards at each clinical center, and all participants provided written informed consent. Per Santa Clara Valley Medical Center policy, this study is exempt from review by the institutional review board because it used publicly available and deidentified data. We followed the strengthening the reporting of observational studies in epidemiology cohort reporting guidelines in presenting our findings. 20
Ascertainment of Incident AF
Ascertainment of AF in the WHI population has been previously described. 14 , 15 Briefly, WHI data have been linked with Medicare data using social security numbers, birth dates, and death dates, with 97% of Medicare‐eligible WHI participants successfully linked. AF incidence was defined as at least a single International Classification of Diseases, Ninth Revision (ICD‐9) AF diagnosis code (427.31) or at least 1 Tenth Revision (ICD‐10) code (I48.0, i48.1x, i48.2x, I49.91) from inpatient, outpatient, or clinician diagnosis while the participant was enrolled in Medicare Fee‐for‐Service Parts A and B (FFS A+B). Participants enrolled in FFS A+B at WHI enrollment entered the risk set at WHI baseline, whereas participants who enrolled in FFS A+B after WHI enrollment were evaluated with a 2‐year look‐back period to assess for preexisting AF at the time of entering the risk set. Participants who were AF‐free for the duration of the look‐back period entered the risk set at the time of completion of the look‐back period. Participants who left FFS A+B were removed from the risk set at the time of their coverage change. Participants who then returned for a subsequent FFS A+B interval were not required to undergo a look‐back period, because they had been established as AF free on their initial entry into the risk set. Because Medicare data were available for some participants at different time periods over WHI follow‐up, a time‐dependent indicator variable of Medicare coverage was added to Cox hazard models described below to adjust for possible ascertainment bias related to differential exposure to Medicare.
Psychosocial Risk Factors and Hierarchical Cluster Analysis
Baseline questionnaires were used to extract self‐reported psychosocial stressors from different domains of life. Eight previously validated psychosocial constructs, including optimism, social support, social strain, stressful life events (SLE), cynical hostility, emotional expressiveness, insomnia, and depressive symptoms, were collected at baseline and included in the analysis as exposure variables. Details of each measure's content, quantification instrument, and reference are described in Table S1.
Because individual psychosocial constructs are not independent, 21 we included all eligible psychosocial constructs available at study baseline and used hierarchical cluster analysis (HCA), an assumption‐free classification analysis tool, to identify homogenous clustering pattern of psychosocial predictors. 22 HCA is advantageous over other techniques, such as factor analysis, in the partitioning of variance. Factor analysis partitions individual variable variance into several factors, whereas HCA assigns the total variance of a variable to a single underlying source based on similarity. 23
In our study, procedure PROC VARCLUS in SAS software was used for HCA. Specifically, maxeigen (the default method with a default value of 1.0) was used to determine clusters. The procedure looks at the eigenvalues between the variables and chooses the cluster with the largest second eigenvalue if it is greater than the maxeigen value. The eigenvalues are the space between clusters, so a higher eigenvalue indicates that a variable is independent from another, whereas a value of 0 is total collinearity.
HCA results were reported according to standard guidelines. 22 , 23 The number of clusters generated by HCA was graphically represented with a dendrogram. To use cluster data in regression analysis, the Z scores for individual variables were computed and averaged to generate composite variables. Measures of central tendency were calculated for each cluster. Clusters were separated into quartiles for the purpose of regression analysis and Kaplan‐Meier survival analysis.
Associations between each exposure variable and the clusters generated from HCA were performed using Pearson correlation coefficient R (Table S2). The (1–R 2) ratio, defined as the ratio of (1–R 2) of each variable in its own cluster over the (1–R 2) of that variable in the other cluster, was computed. The lower the value of the ratio, the better fit a given variable is within that cluster and is used to identify how well a variable is performing within its cluster.
Assessment of the association between continuous HCA clusters and AF was done using Cox proportional hazards models in a series of hierarchal adjustments, with results presented as hazard ratios (HRs) and 95% CIs. In addition to unadjusted models, we looked at models adjusted for age, ethnicity, race, and education (Model 1). Then, additional adjustment for waist–hip ratio, physical activity, smoking, and alcohol (Model 2), and finally additional adjustment for hypertension, diabetes, history of heart failure, and history of myocardial infarction (Model 3). 14 , 15 In addition to these adjustments, all models were stratified within the model by WHI component (clinical trial/observational study). All exposures of interest had the proportional hazards assumption verified by testing the interaction of follow‐up and the exposure variables as well as through visual inspection of the log‐likelihood plot of developing AF over time.
The relationships between quartiles of each cluster and AF were assessed using the same hierarchal adjustment methods used with the continuous cluster modeling. Kaplan‐Meier curves of AF by quartiles of each cluster were presented with events and number at risk over the follow‐up period.
Sensitivity Analyses
To address the potential for ascertainment bias (ie, participants with psychosocial stressors may be more likely to seek medical attention and therefore more likely to be diagnosed with AF), we adjusted the primary model (Model 3) for the number of inpatient and outpatient claims per year before AF diagnosis. Second, to address the possibility that sleep apnea or other sleep disorders could confound the association between insomnia and AF, a sensitivity analysis was performed using snoring as a surrogate marker (stratified as <1 night per week, 1+ nights per week, and do not know), because information on sleep disorders was not collected in WHI participants.
Subgroup Analyses
To evaluate whether the relationship between the continuous clusters and AF differed by baseline characteristics, we examined subgroup analyses with Cox proportional hazards models with AF as a function of the continuous clusters, the subgroup of interest, and their interaction. Racial and ethnic subgroups were limited to those racial and ethnic groups with at least 1% of the overall sample. These models were adjusted for the full covariate list above as well as the cohorts for heart and aging research in genomic epidemiology for atrial fibrillation (CHARGE‐AF) risk score, dichotomized into 5‐year risk of AF of <5% (low risk) and ≥5% (high risk) categories in our study to represent traditional risk factors for the purposes of secondary analyses. The CHARGE‐AF score was developed in 3 US community‐based studies and validated in 2 large European community‐based studies 24 , 25 , 26 , 27 to predict incident AF within 5 years in diverse patient populations. It is calculated as:
0.508×age (5 years) +0.465 (White)+0.248×height (10 cm)+0.115×weight (15 kg)+0.197×systolic blood pressure (20 mm Hg) − 0.101×diastolic blood pressure (10 mm Hg)+0.359×current smoker +0.349×antihypertensive medication+0.237×diabetes +0.701×heart failure +0.496×myocardial infarction.
Statistical Analysis
Baseline characteristics of the sample are presented with means and standard deviations for continuous variables and with frequencies and percentages for categorical variables. Self‐reported race and ethnicity were included in the subgroup analysis given the observed race‐ and ethnicity‐based differences in AF incidence, lifetime stroke risk, mortality, symptoms, and quality of life, as well as treatment strategies. 28 P values were 2‐sided and considered significant at values <0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Population Characteristics
Of the 106 784 participants who had Medicare follow‐up data, 4367 (4.1%) with AF at baseline were excluded, and an additional 18 681 (17.5%) were excluded due to missing data, leaving 83 736 participants in the final study cohort. Baseline participant characteristics, including demographic and medical history, as well as standardized scores for the 8 psychosocial predictors are summarized in Table 1. Participants were 63.9±7.0 years of age, 73 798 (88.1%) were White, 2411 (2.9%) were Hispanic, and 5999 (7.2%) were Black. Over an average follow‐up duration of 10.5±6.2 years, there were 23 954 (28.6%) participants with incident AF.
Table 1.
Baseline Characteristics (n=83 736) by Low and High Cluster Quartiles
| Characteristic | All participants (n=83 736) | Stress Cluster | Strain Cluster | ||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 4 | Quartile 1 | Quartile 4 | ||
| Demographics | |||||
| Age, mean (SD) | 63.9 (7.0) | 63.9 (6.8) | 63.2 (7.1) | 63.9 (6.9) | 63.5 (7.1) |
| Ethnicity | |||||
| Not Hispanic or Latino | 80 822 (96.5) | 20 328 (97.2) | 19 877 (95.2) | 20 410 (97.5) | 19 825 (94.7) |
| Hispanic or Latino | 2411 (2.9) | 454 (2.2) | 862 (4.1) | 434 (2.1) | 926 (4.4) |
| Unknown/not reported | 503 (0.6) | 122 (0.6) | 149 (0.7) | 88 (0.4) | 184 (0.9) |
| Race | |||||
| American Indian or Alaska Native | 220 (0.3) | 41 (0.2) | 93 (0.4) | 38 (0.20) | 87 (0.4) |
| Asian | 1828 (2.2) | 640 (3.1) | 310 (1.5) | 354 (1.7) | 526 (2.5) |
| Native Hawaiian or Other Pacific Islander | 55 (0.1) | 6 (0.0) | 19 (0.1) | 8 (0.0) | 21 (0.1) |
| Black | 5999 (7.2) | 1350 (6.5) | 1853 (8.9) | 1157 (5.5) | 2120 (10.1) |
| White | 73 798 (88.1) | 18 566 (88.8) | 17 924 (85.8) | 19 059 (91.1) | 17 482 (83.5) |
| >1 race | 906 (1.1) | 141 (0.7) | 333 (1.6) | 169 (0.8) | 308 (1.5) |
| Unknown/not reported | 930 (1.1) | 160 (0.8) | 356 (1.7) | 147 (0.7) | 391 (1.9) |
| Body mass index, km/m2, mean (SD) | 27.8 (5.8) | 27.1 (5.5) | 28.7 (6.2) | 27.1 (5.4) | 28.9 (6.3) |
| <25 | 29 832 (35.6) | 8377 (40.1) | 6352 (30.4) | 8460 (40.4) | 6152 (29.4) |
| 25–<30 | 29 121 (34.8) | 7260 (34.7) | 7032 (33.7) | 7298 (34.9) | 7086 (33.8) |
| 30–<35 | 15 081 (18.0) | 3387 (16.2) | 4313 (20.6) | 3339 (16.0) | 4337 (20.7) |
| 35–<40 | 6010 (7.2) | 1184 (5.7) | 1943 (9.3) | 1141 (5.5) | 2041 (9.7) |
| ≥40 | 3021 (3.6) | 535 (2.6) | 1075 (5.1) | 523 (2.5) | 1149 (5.5) |
| Waist–hip ratio, mean (SD) | 0.81 (0.08) | 0.80 (0.08) | 0.82 (0.08) | 0.80 (0.08) | 0.82 (0.08) |
| Education | |||||
| High school/GED or less | 17 767 (21.2) | 3691 (17.7) | 5304 (25.4) | 3322 (15.9) | 5852 (28.0) |
| After high school | 31 180 (37.2) | 7167 (34.3) | 8364 (40.0) | 7258 (34.7) | 8297 (39.6) |
| College degree or higher | 34 789 (41.5) | 10 046 (48.1) | 7220 (34.6) | 10 352 (49.5) | 6786 (32.4) |
| Income, US$ | |||||
| <$20 000 | 11 476 (13.7) | 1986 (9.5) | 4222 (20.2) | 1741 (8.3) | 4671 (22.3) |
| $20 000–$49 999 | 34 963 (41.8) | 8297 (39.7) | 8887 (42.5) | 7974 (38.1) | 8983 (42.9) |
| $50 000–$74 999 | 16 195 (19.3) | 4484 (21.5) | 3501 (16.8) | 4551 (21.7) | 3355 (16.0) |
| ≥$75 000 | 16 293 (19.5) | 4950 (23.7) | 3062 (14.7) | 5493 (26.2) | 2657 (12.7) |
| WHI component | |||||
| Clinical trial | 34 081 (40.7) | 8212 (39.3) | 8745 (41.9) | 8380 (40.0) | 8603 (41.1) |
| Observational study | 49 655 (59.3) | 12 692 (60.7) | 12 143 (58.1) | 12 552 (60.0) | 12 332 (58.9) |
| Medical history | |||||
| Hypertension | 24 686 (29.5) | 5340 (25.5) | 6846 (32.8) | 5432 (26.0) | 7002 (33.4) |
| Diabetes | 3320 (4.0) | 605 (2.9) | 1155 (5.5) | 595 (2.8) | 1296 (6.2) |
| Hyperlipidemia | 11 598 (13.9) | 2588 (12.4) | 3232 (15.5) | 2545 (12.2) | 3296 (15.7) |
| CAD | 2249 (2.7) | 385 (1.8) | 771 (3.7) | 431 (2.1) | 752 (3.6) |
| MI | 1567 (1.9) | 257 (1.2) | 559 (2.7) | 298 (1.4) | 545 (2.6) |
| CABG/PTCA | 1281 (1.5) | 225 (1.1) | 430 (2.1) | 252 (1.2) | 424 (2.0) |
| Stroke /TIA | 2190 (2.6) | 435 (2.1) | 714 (3.4) | 408 (1.9) | 734 (3.5) |
| HF | 751 (0.9) | 108 (0.5) | 279 (1.3) | 119 (0.6) | 291 (1.4) |
| PAD | 1491(1.8) | 237(1.1) | 589 (2.8) | 275 (1.3) | 569 (2.7) |
| Smoking | |||||
| Never | 42 736 (51.0) | 11 199 (53.6) | 10 136 (48.5) | 11 288 (53.9) | 10 270 (49.1) |
| Past | 35 476 (42.4) | 8474 (40.5) | 9000 (43.1) | 8573 (41.0) | 8833 (42.2) |
| Current | 5524 (6.6) | 1231 (5.9) | 1752 (8.4) | 1071 (5.1) | 1832 (8.8) |
| Alcohol use, No. of drinks per wk | |||||
| 0 | 34 272 (40.9) | 8304 (39.7) | 9376 (44.9) | 7894 (37.7) | 9978 (47.7) |
| >0–<7 | 39 229 (46.8) | 9961 (47.7) | 9178 (43.9) | 10 085 (48.2) | 8978 (42.9) |
| ≥7 | 10 235 (12.2) | 2639 (12.6) | 2334 (11.2) | 2953 (14.1) | 1979 (9.5) |
| Physical activity, MET h per wk, mean (SD) | 12.6 (13.7) | 14.1 (14.5) | 10.9 (13.1) | 14.4 (14.5) | 10.7 (12.9) |
| Psychosocial stressors, mean (SD) | |||||
| Optimism | 23.4 (3.4) | 24.6 (3.0) | 21.8 (3.7) | 26.2 (2.4) | 20.4 (3.3) |
| Social support | 36.2 (7.6) | 38.6 (6.5) | 33.0 (8.4) | 41.4 (4.2) | 29.8 (8.0) |
| Social strain | 6.5 (2.5) | 5.6 (2.0) | 7.7 (2.8) | 4.8 (1.2) | 8.8 (2.7) |
| SLE | 3.3 (3.1) | 1.0 (1.2) | 6.2 (3.8) | 2.3 (2.4) | 4.6 (3.8) |
| Cynical hostility | 3.6 (2.8) | 2.8 (2.5) | 4.5 (3.0) | 1.5 (1.6) | 6.1 (2.7) |
| Emotional expressiveness | 5.8 (1.0) | 5.6 (0.9) | 6.1 (1.0) | 5.1 (0.8) | 6.5 (0.9) |
| Depressive symptoms | 2.3 (2.5) | 0.4 (0.7) | 5.1 (2.9) | 1.2 (1.6) | 3.9 (3.1) |
| Insomnia | 6.6 (4.4) | 2.6 (1.9) | 11.1 (4.2) | 5.2 (4.0) | 8.2 (4.8) |
Values are n (%) or mean (SD). CABG indicates coronary artery bypass graft; CAD, coronary artery disease; GED, general educational development; HF, heart failure; MET, metabolic equivalent of task; MI, myocardial infarction; PAD, peripheral artery disease; PTCA, percutaneous transluminal angioplasty; SLE, stressful life events; TIA, transient ischemic attack; and WHI, Women's Health Initiative.
Forming Psychosocial Clusters
All standardized psychosocial predictors were entered into the HCA. Two clusters were generated as the optimal solution with second eigenvalue for both clusters <1.0 as set by the maxeigen procedure default. The proportion of total variance explained by clustering improved from 0.34 with the 1‐cluster solution to 0.46 with the 2‐cluster solution (Figure 1).
Figure 1. Dendrogram of grouping individual psychosocial constructs into 2 clusters by hierarchical cluster analysis.
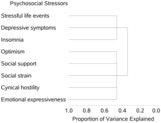
The Stress Cluster included SLE, depressive symptoms, and insomnia. The Strain Cluster included optimism, social support, social strain, cynical hostility, and emotional expressiveness. Baseline characteristics of the study cohort based on the 2 clusters' first and fourth quartiles are summarized in Table 1.
Pearson statistics (correlation coefficient, R) and their corresponding P values of correlation between standardized psychosocial predictors with and within their respective clusters are summarized in Table S2. All of the predictors were strongly correlated with their respective cluster with Pearson correlation coefficient (R) ranging from 0.58 (emotional expressiveness within the Strain Cluster) to 0.82 (depressive symptoms within the Stress Cluster) (P<0.001). Within the Stress Cluster, depressive symptoms and insomnia were the factors that were most closely correlated with each other, with an R of 0.47 (P<0.001). Depressive symptoms were the strongest predictor within the Stress Cluster with (1–R 2) ratio of 0.36, whereas lack of optimism was the strongest predictor of the Strain Cluster with (1–R 2) ratio of 0.58.
Individual Psychosocial Predictors and Incident AF
Cox hazard regression modeling of the 8 standardized psychosocial predictors in separate models or combined into 1 multivariate model predicting incident AF while adjusting for known AF risk factors is presented in Table 2. On univariate analyses, all 8 psychosocial predictors were significantly associated with incident AF. The associations, however, were attenuated after adjustments for the other 7 standardized psychosocial constructs as well as age, ethnicity, race, education, WHR, physical activity, smoking, alcohol, hypertension, diabetes, heart failure, and myocardial infarction. Most notably, the association between depressive symptoms and AF was no longer statistically significant (HR, 1.00 per unit score [95% CI, 0.98–1.01]), whereas SLE and insomnia remained statistically significant in the combined multivariate model. In the Strain Cluster, only social strain retained a marginal statistical significance (HR, 1.02 [95% CI, 1.00–1.03]), whereas optimism, social support, cynical hostility, and emotional expressiveness were no longer statistically significantly associated with incident AF.
Table 2.
Univariable and Multivariate Adjusted Hazard Ratios of Each Component Psychosocial Construct* as Well as Continuous Psychosocial Clusters on Incident Atrial Fibrillation
| Construct | Individual† | Combinedǂ | ||
|---|---|---|---|---|
| HR (95% CI)§ | P value | HR (95% CI)§ | P value | |
| Stressful life events | 1.04 (1.03–1.05) | <0.001 | 1.02 (1.01–1.04) | <0.001 |
| Depressive symptoms | 1.04 (1.02–1.05) | <0.001 | 1.00 (0.98–1.01) | 0.85 |
| Insomnia | 1.05 (1.04–1.06) | <0.001 | 1.04 (1.03–1.06) | <0.001 |
| Optimismǁ | 1.02 (1.01–1.04) | <0.001 | 1.00 (0.99–1.02) | 0.56 |
| Social supportǁ | 1.03 (1.01–1.04) | <0.001 | 1.01 (1.00–1.02) | 0.16 |
| Social strain | 1.04 (1.02–1.05) | <0.001 | 1.02 (1.00–1.03) | 0.02 |
| Cynical hostility | 1.02 (1.01–1.03) | 0.003 | 1.00 (0.99–1.02) | 0.71 |
| Emotional expressiveness | 1.02 (1.00–1.03) | 0.02 | 1.00 (0.99–1.01) | 0.99 |
| Clusters¶ | Unadjusted | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI)# | P value | HR (95% CI)# | P value | HR (95% CI)# | P value | HR (95% CI)# | P value | |
| Stress | 1.07 (1.05–1.09) | <0.001 | 1.09 (1.07–1.11) | <0.001 | 1.08 (1.06–1.10) | <0.001 | 1.07 (1.05–1.09) | <0.001 |
| Strain | 1.04 (1.02–1.07) | <0.001 | 1.06 (1.03–1.08) | <0.001 | 1.04 (1.02–1.06) | 0.001 | 1.03 (1.00–1.05) | 0.02 |
All models are stratified by Women's Health Initiative component (clinical trial/observational study). Model 1: Adjusted for age, ethnicity, race, and education. Model 2: Model 1 + waist–hip ratio, physical activity, smoking, and alcohol use. Model 3: Model 2 + hypertension, diabetes, heart failure, and myocardial infarction. Stress Cluster: stressful life events, depressive symptoms, insomnia. Strain Cluster: Optimism, social support, social strain, cynical hostility, emotional expressiveness. HR indicates hazard ratio.
HRs and CIs are from a proportional hazards model with incident atrial fibrillation as a function of standardized constructs. Models are stratified by the Women's Health Initiative component (clinical trial/observational study) and are adjusted for age, ethnicity, race, education, waist–hip ratio, physical activity, smoking, alcohol use, hypertension, diabetes, heart failure, and myocardial infarction.
Atrial fibrillation modeled separately by each individual standardized construct.
Atrial fibrillation modeled by all standardized constructs in 1 proportional hazards model.
HRs, corresponding CIs, and P values are for an increase of 1 point in the given standardized construct score.
Standardized psychosocial stressor values are inverted.
HRs and CIs are from a proportional hazards model with incident atrial fibrillation as a function of continuous Stress and Strain Clusters.
HRs, corresponding CIs, and P values are for an increase of 1 point in the given cluster score.
Psychosocial Clusters and Incident AF
Univariate and multivariable Cox proportional hazard regression analyses of the associations between the 2 psychosocial clusters and incident AF are reported in Table 2. Both clusters were significantly associated (P<0.001) with incident AF in the unadjusted models and remained significantly associated with incident AF after adjusting for covariates including age, ethnicity, race, education, waist–hip ratio, physical activity, smoking, alcohol, hypertension, diabetes, heart failure, and myocardial infarction. Using the fully adjusted Cox hazard regression model (Model 3), a higher value in the Stress Cluster was associated with higher incidence of AF (HR, 1.07 per unit value of cluster score [95% CI, 1.05–1.09]). In the fully adjusted model, a higher value in the Strain Cluster was also associated with higher AF incidence (HR, 1.03 per unit value of cluster score [95% CI, 1.00–1.05]).
When the standardized cluster scores were divided into quartiles, in a fully adjusted Cox hazard regression model, those in the highest quartile in the Stress Cluster had a higher rate of incident AF compared with those in the lowest quartile (HR, 1.14 [95% CI, 1.10–1.19]). Similarly, those in the highest quartile in the Strain Cluster had a higher rate of incident AF compared with those in the lowest quartile (HR, 1.03 [95% CI, 1.00–1.08]; Table S3). Freedom from AF over time as a function of cluster quartiles is illustrated in the Kaplan‐Meier plots (the Stress Cluster in Figure 2A and the Strain Cluster in Figure 2B).
Figure 2. Kaplan‐Meier AF‐free survival by quartiles of the Stress Cluster (A) and the Strain Cluster (B).
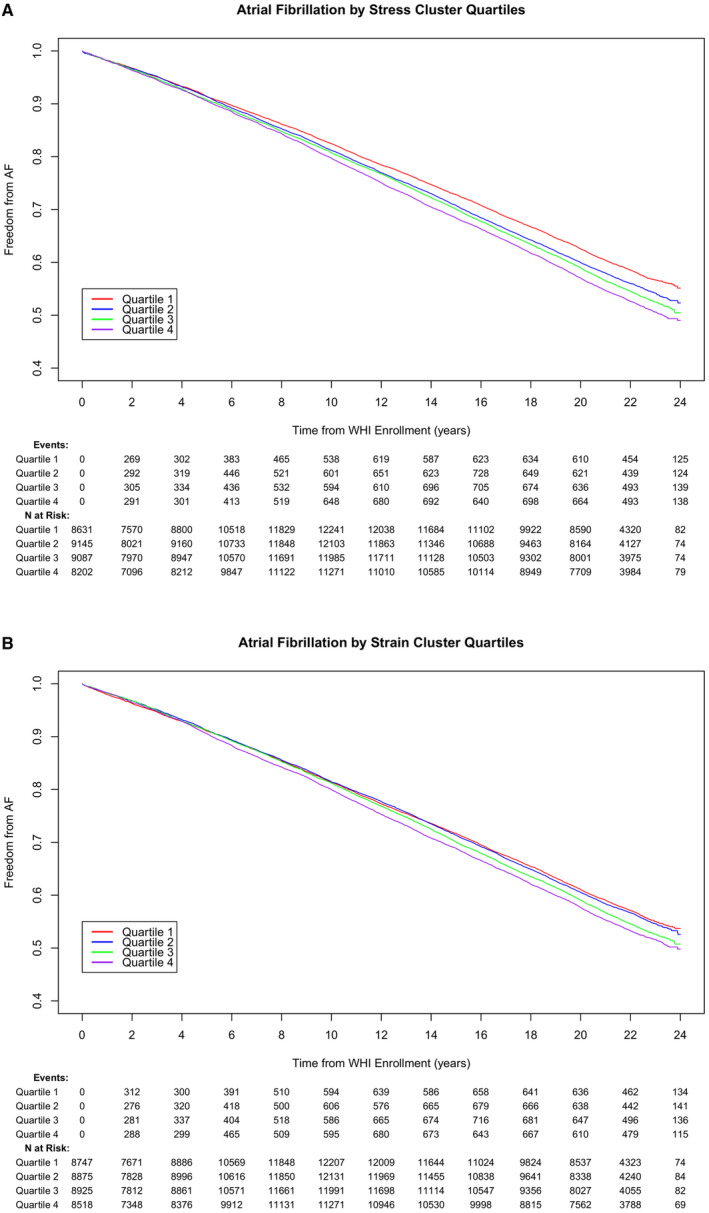
AF indicates atrial fibrillation; and WHI, Women's Health Initiative.
Sensitivity Analyses
The average number of inpatient/outpatient claims per years before AF diagnosis was 4.0±4.5 during their Medicare follow‐up. Upon adjusting this variable in the primary model (Model 3), the associations between the Stress and Strain Cluster and incident AF remained significant (HR, 1.06 [95% CI, 1.04–1.08]; HR, 1.03 [95% CI, 1.00–1.05], respectively).
To evaluate if the association between the Stress Cluster, which contained insomnia, and AF may be confounded by sleep apnea, we conducted a sensitivity analysis controlling for self‐reported snoring, which did not significantly change Model 3 findings (HR, 1.07 [95% CI, 1.05–1.09]; HR, 1.03 [95% CI, 1.00–1.05], for the Stress and Strain Cluster, respectively).
Subgroup Analyses
Subgroup analyses were performed to assess for interactions between the 2 psychosocial clusters and traditional AF risk factors. There were no significant interactions between the 2 psychosocial clusters on incident AF with regard to alcohol consumption, hypertension, diabetes, or coronary artery disease (Table S4 and Figure 3).
Figure 3. Continuous psychosocial clusters on incident atrial fibrillation (AF), stratified by baseline subgroups.
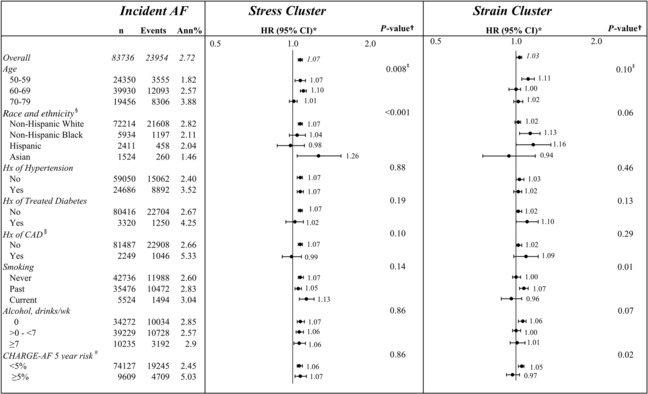
Subgroup hazard ratios (HRs), 95% CIs, and interaction P values are from a proportional hazards regression, with AF as a function of the Stress Cluster or the Stress Cluster by subgroup interaction, the Strain Cluster, and the Strain Cluster by subgroup interaction, stratified by Women's Health Initiative (WHI) component (clinical trial/observational study), and adjusted for age, ethnicity, race, education, waist–hip ratio (WHR), physical activity, smoking, alcohol use, hypertension, diabetes, heart failure, and myocardial infarction (MI). *HRs and corresponding CIs are for an increase of 1 point in the given cluster score. † P value is for the subgroup by cluster interaction. ǂCluster by age interaction terms from a separate model with linear trend over age groups, the Stress Cluster, Stress Cluster by linear trend over age groups interaction, the Strain Cluster, and Strain Cluster by linear trend over age groups interaction. § White=non‐Hispanic White; Black=non‐Hispanic Black; Hispanic=Hispanic, all races; Asian=non‐Hispanic Asian. ǁHistory (Hx) of coronary artery disease (CAD) subgroup model is not adjusted for history of MI. #Cohorts for heart and aging research in genomic epidemiology for atrial fibrillation (CHARGE‐AF) (a validated score 24 , 25 , 26 , 27 encompassing traditional AF risk factors to predict incident AF within 5 years in diverse patient populations) subgroup model is stratified by WHI component (clinical trial/observational study) and adjusted for ethnicity, race, education, WHR, physical activity, and alcohol use. Ann%, annual percent event rate.
For the Stress Cluster, we found a significant interaction with age, with higher HRs in the younger age groups 60 to 69 years (HR, 1.10 [95% CI, 1.07–1.13]) and 50 to 59 years (HR, 1.07 [95% CI, 1.02–1.12]) than the 70 to 79 years age group (HR, 1.01 [95% CI, 0.97–1.04]) (P for interaction=0.008).
The Stress Cluster was also found to have a significant interaction with different racial groups, with higher HRs in non‐Hispanic White (HR, 1.07 [95% CI, 1.05–1.09]) and Asian women (HR, 1.26 [95% CI, 1.04–1.54]) than Hispanic (HR, 0.98 [95% CI, 0.87–1.11]) and non‐Hispanic Black women (HR, 1.04 [95% CI, 0.97–1.12]) (P for interaction<0.001).
The CHARGE AF score, dichotomized as a 5‐year risk of AF of <5% (low) and ≥5% (high) risk categories, served as a marker for overall risk of AF. There was no significant interaction between CHARGE AF risk and the Stress Cluster (P for interaction=0.86). We did find a significant interaction between the CHARGE AF score in the Strain Cluster, such that in those with a low CHARGE‐AF score, there was a higher association between the Strain Cluster and incident AF compared with those with a higher CHARGE‐AF score (HR, 1.05 versus 0.97; P for interaction=0.02).
Discussion
In this large cohort of postmenopausal women from the WHI followed for >10 years, 8 individual psychosocial factors were grouped into 2 distinct clusters by HCA. Both the Stress Cluster (SLE, depressive symptoms, and insomnia) and the Strain Cluster (optimism, cynical hostility, emotional expressiveness, social support, and social strain) were significantly associated with incident AF after controlling for traditional AF risk factors. Higher insomnia and SLE scores had the strongest independent associations with incident AF on Cox hazard regression analyses. These results support our hypothesis that psychosocial predictors accounted for additional risk of AF above and beyond traditional AF risk factors.
AF is a complex cardiac arrhythmia that results from the interplay of varied genetic, environmental, biological, and lifestyle factors. 29 Although AF incidence is lower in women, due to increased longevity and higher numbers of women, AF affects as many women as men. Women with AF were also found to have worse outcomes in terms of stroke and mortality compared to men. 4 , 6 Identifying and addressing sex‐specific, modifiable risk factors, therefore, may help reduce the burden of AF in aging women. Emotional distress and affective disorders have been proposed as potential AF risk factors because they affect the autonomous nervous system, hypothalamus–pituitary–adrenal axis, and renin–angiotensin–aldosterone system, mechanisms that have been involved in the pathogenesis of AF. 8 , 9 , 30 Women have a higher prevalence of depressive symptoms, posttraumatic stress disorder, higher stress vulnerability, and exposure to stress than men. 31 The findings from studies assessing the association between psychosocial predictors and AF, however, have been mixed. The Framingham Offspring Study found that tension was associated with a higher risk for AF in men, but not in women, whereas there was no associations with anxiety in either men or women. 11 In the MESA (Multi‐Ethnic Study of Atherosclerosis) cohort, depressive symptoms, but not anxiety, anger, or chronic stress, were associated with an increased risk for AF. 32 Neither psychological distress (as measured by anxiety, depression, low affective activation) nor depression alone were associated with AF risk in the Women's Health Study of female health professionals. 12
The different populations studied and different psychosocial domains being evaluated could explain these inconsistencies. Furthermore, most studies modeled 1 or several psychosocial characteristics at a time in search of independent effects. This approach may overlook the interrelationships among the various factors with considerable construct and measurement overlap. 21 Individual risk may be modeled more accurately using composite measures that pool information across variables that each tap into 1 facet of a potential underlying structure. Therefore, in our study, HCA was used to model the panel of candidate psychosocial variables into clusters, wherein both homogeneity within clusters as well as heterogeneity between clusters were maximized, rather than using individual constructs.
As far as we know, our study is the first to use HCA to analyze the association between AF and psychosocial risk factors. From the existing evidence about psychosocial clusters, Frasure‐Smith and Lespérance 33 and Clark et al 34 reported on a positive cluster and a negative cluster with associated health outcomes in the expected direction. Similarly, Jabson et al, in their study based on WHI data, also identified a Social Cluster (positive cluster) and a Stress Cluster (negative cluster) in association with well‐being and health. 35 Distinct from the negative/positive framework as outlined by the above studies, our Strain and Stress Clusters bear closer similarities to the classification system by Kop et al, who proposed 3 types of psychosocial stressors in promoting coronary disease progression and cardiac ischemia: the chronic, negative affective factors (such as cynical hostility and anxiety, which tend to show considerable stability across years and can be considered dispositional), the episodic factors (major depressive episodes), and the acute risk factors (eg, SLE). 36 The differential effects of acute versus chronic psychosocial stress on myocardial ischemic injury has been recognized in both experimental models and clinical cohorts but has not been previously examined in the context of AF. 37
Of the 3 individual components of the Stress Cluster, insomnia, depressive symptoms, and SLE all showed strong association with AF. The advantage of HCA over traditional analytic strategies with the assumption of independent effects of candidate variables was illustrated here by the 2 closely correlated variables, depressive symptoms and insomnia, with depressive symptoms being rejected in the Cox model but retained in the HCA model. With the HSA method, the clustering of psychosocial risk factors working synergistically on the condition is recognized, rather than evaluating and then rejecting interconnected and overlapping variables based on individual statistical probability.
Insomnia, a factor strongly and independently associated with AF among all 8 psychosocial constructs, could be a key mediator for the link between depressive symptoms and other predictors and AF. There is a growing body of data suggesting the quality and quantity of sleep may affect AF, beyond the well‐established association between obstructive sleep apnea and AF. 38 , 39 , 40 , 41 , 42 In our analysis, a sensitivity analysis using snoring as a surrogate marker (because the WHI did not collect sleep apnea data at baseline) did not change the outcome of the main HCA model, supporting the independent effect of insomnia on AF. With a baseline prevalence of 25% in WHI women, 43 insomnia is a potential modifiable target for reducing the risk of AF through both behavioral and pharmacological interventions.
Although chronic stress has not been consistently associated with AF, 12 , 44 acute stressful events in life, as captured by the SLE construct in this analysis, was 1 of the 3 components of the Stress Cluster that was most strongly associated with incident AF. It should be noted that the SLE construct in WHI not only captured the exposure to the 11 types of SLEs, but also the degree of emotional distress (from 0=no to 3=yes, the event upsets me very much) associated with the events. This construct has been associated with cardiovascular disease 45 and coronary artery disease risk in women with diabetes. 46 Other psychophysiological factors associated with the exposure to an SLE, beyond the event itself, may also be a mechanism affecting AF risk in these women, and this should be studied further in future research.
The Strain Cluster encompassed 3 personality traits (optimism, cynical hostility, and emotional expressiveness, with emotional expressiveness representing both negative emotional expressiveness and ambivalence over emotional expressiveness) and 2 social measures (social support and social strain). The clustering of these 2 groups of constructs was more than statistical, reflecting the long‐recognized interrelationship between personality and social interactions conceptualized by Mischel and Shoda. 47 Several pathways were suggested, including: (1) People tend to select and create their social environment to be consistent with their personality traits. (2) Personality traits may evoke supportive and unsupportive reactions from others. (3) Personality traits may modify how social support is evaluated. 48 The effect size of the Strain Cluster on AF, though significant, was small. An in‐depth understanding of these chronic negative affectivity traits and social strain measures in the onset and progression of AF may potentially aid in prioritizing resources in optimizing treatment and quality of life in patients with AF.
The subgroup analyses explored the interaction of psychosocial risk factors with traditional AF risk factors individually as well as a marker of global atherosclerosis burden. Most notably, the Stress Cluster had a stronger association with AF incidence in younger women (50–69 versus 70–79 years of age), whereas the Strain Cluster was associated with higher AF risk in those with low CHARGE‐AF scores. This is in support of our second hypothesis that the younger the patient, and the lower the overall atherosclerosis burden, psychosocial factors may feature more prominently in promoting AF. The finding that the Stress Cluster played a stronger role in non‐Hispanic White or Asian women, however, needs to be interpreted with caution. The WHI sample selection limits the interpretation of findings to the overall US population or racial or ethnic subpopulations identified. 49 AF is imbedded within a societal context in which socioeconomic and environmental social factors may also affect the onset of AF, apart from mental psychosocial stress factors, and could be due to chance given the high number of tests conducted. Corroboration from data collected from large, diverse cohorts are needed to further investigate the possible differential effect of psychosocial risks in different age, race and ethnicity, and background atherothrombotic risk groups.
The large sample size, the long follow‐up period, and the validated methods of classifying incident AF were notable strengths of this study, along with the detailed characterization of the baseline psycho–social–behavioral profile of study participants. Our findings add to the growing understanding of how psychosocial predictors interrelate and how empirically formed risk clusters associate with health outcomes. Distinct from previous studies that reported the positive or null association of individual stressors with AF, our results provide a unique approach for incorporating multiple, interrelated psychosocial risk factors into 2 clusters, representing chronic dispositional stress (the Strain Cluster) versus more acute/episodic stress (the Stress Cluster). Intervention strategies may be tailored to improve clinical outcomes and cost‐effectiveness based on an individual's Stress and Strain Cluster profile, with special attention to the timeliness of intervention to address acute SLE and insomnia (the 2 strongest factors associated with AF).
There were, however, several limitations in this study. First, although psychosocial variables may change over time, the psychometric questionnaires were administered only at study entry. Given the long follow‐up duration, time‐varying assessments would capture more adequately the cumulative role of stress exposure and coping behaviors in the development of AF, but these were not available longitudinally. Although sleep apnea and other sleep disorders may confound the relationship between insomnia and AF, data on these disorders are not available in this cohort. Although a sensitivity analysis controlling for snoring as a proxy for sleep disorders is presented, we acknowledge that this is an imperfect surrogate for sleep apnea. Furthermore, generalizability to other demographic, racial, and ethnic groups is limited, because the study was conducted primarily in postmenopausal White (≈90%) women. Lastly, causal associations cannot be inferred based on this retrospective cohort analysis alone, and unmeasured or inadequately measured confounders may explain the observed associations.
Conclusions
Our findings add to the growing body of evidence showing a close association between AF and the spectrum of psychosocial risk factors as grouped in the Stress Cluster and the Strain Cluster, highlighting the important role of mental health–related risk factors in AF pathophysiology and strategies for risk modification. Further studies elucidating the relationship and mitigating the risks of chronic exposure to psychosocial stressors and AF are warranted.
Sources of Funding
The Women's Health Initiative program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005.
Disclosures
None.
Supporting information
Tables S1–S4
References 50–60
Acknowledgments
The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A listing of WHI investigators can be found at https://www.whi.org/doc/WHI‐Investigator‐Short‐List.pdf.
This article was sent to Daniel T. Eitzman, MD, Senior Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030030
For Sources of Funding and Disclosures, see page 12.
REFERENCES
- 1. Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, Noseworthy PA, Perez MV, Turakhia MP; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council . Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation. 2018;137:e623–e644. doi: 10.1161/CIR.0000000000000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11:639–654. doi: 10.1038/nrcardio.2014.118 [DOI] [PubMed] [Google Scholar]
- 3. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, Odutayo AA. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta‐analysis of cohort studies. BMJ. 2016;532:h7013. doi: 10.1136/bmj.h7013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ko D, Rahman F, Schnabel RB, Yin X, Benjamin EJ, Christophersen IE. Atrial fibrillation in women: epidemiology, pathophysiology, presentation, and prognosis. Nat Rev Cardiol. 2016;13:321–332. doi: 10.1038/nrcardio.2016.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14:195–203. doi: 10.11909/j.issn.1671-5411.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 8. Patel D, Mc Conkey ND, Sohaney R, Mc Neil A, Jedrzejczyk A, Armaganijan L. A systematic review of depression and anxiety in patients with atrial fibrillation: the mind‐heart link. Cardiovasc Psychiatry Neurol. 2013;2013:159850. doi: 10.1155/2013/159850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peacock J, Whang W. Psychological distress and arrhythmia: risk prediction and potential modifiers. Prog Cardiovasc Dis. 2013;55:582–589. doi: 10.1016/j.pcad.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polikandrioti M, Koutelekos I, Vasilopoulos G, Gerogianni G, Gourni M, Zyga S, Panoutsopoulos G. Anxiety and depression in patients with permanent atrial fibrillation: prevalence and associated factors. Cardiol Res Pract. 2018;2018:7408129. doi: 10.1155/2018/7408129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eaker ED, Sullivan LM, Kelly‐Hayes M, D'Agostino RB, Benjamin EJ. Tension and anxiety and the prediction of the 10‐year incidence of coronary heart disease, atrial fibrillation, and total mortality: the Framingham Offspring Study. Psychosom Med. 2005;67:692–696. doi: 10.1097/01.psy.0000174050.87193.96 [DOI] [PubMed] [Google Scholar]
- 12. Whang W, Davidson KW, Conen D, Tedrow UB, Everett BM, Albert CM. Global psychological distress and risk of atrial fibrillation among women: the Women's Health Study. J Am Heart Assoc. 2012;1:e001107. doi: 10.1161/JAHA.112.001107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Westcott SK, Beach LY, Matsushita F, Albert CM, Chatterjee N, Wong J, Williams DR, Vinayagamoorthy M, Buring JE, Albert MA. Relationship between psychosocial stressors and atrial fibrillation in women >45 years of age. Am J Cardiol. 2018;122:1684–1687. doi: 10.1016/j.amjcard.2018.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perez MV, Wang PJ, Larson JC, Soliman EZ, Limacher M, Rodriguez B, Klein L, Manson JE, Martin LW, Prineas R, et al. Risk factors for atrial fibrillation and their population burden in postmenopausal women: the Women's Health Initiative observational study. Heart. 2013;99:1173–1178. doi: 10.1136/heartjnl-2013-303798 [DOI] [PubMed] [Google Scholar]
- 15. Perez MV, Wang PJ, Larson JC, Virnig BA, Barbara C, David CJ, Liviu K, Manson JAE, Martin LW, Jennifer R, et al. Effects of postmenopausal hormone therapy on incident atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:1108–1116. doi: 10.1161/CIRCEP.112.972224 [DOI] [PubMed] [Google Scholar]
- 16. Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–S77. doi: 10.1016/S1047-2797(03)00042-5 [DOI] [PubMed] [Google Scholar]
- 17. Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative observational study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–S121. doi: 10.1016/S1047-2797(03)00047-4 [DOI] [PubMed] [Google Scholar]
- 18. Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women's Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S78–S86. doi: 10.1016/S1047-2797(03)00045-0 [DOI] [PubMed] [Google Scholar]
- 19. Design of the Women's Health Initiative clinical trial and observational study . The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/S0197-2456(97)00078-0 [DOI] [PubMed] [Google Scholar]
- 20. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 21. Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260 [DOI] [PubMed] [Google Scholar]
- 22. Clatworthy J, Buick D, Hankins M, Weinman J, Horne R. The use and reporting of cluster analysis in health psychology: a review. Br J Health Psychol. 2005;10:329–358. doi: 10.1348/135910705X25697 [DOI] [PubMed] [Google Scholar]
- 23. Blashfield RK. Propositions regarding the use of cluster analysis in clinical research. J Consult Clin Psychol. 1980;48:456–459. doi: 10.1037/0022-006X.48.4.456 [DOI] [PubMed] [Google Scholar]
- 24. Shulman E, Kargoli F, Aagaard P, Hoch E, Biase LD, Fisher JD, Gross JN, Kim SG, Krumerman AK, Ferrick KJ. Validation of the Framingham Heart Study and CHARGE‐AF risk scores for atrial fibrillation in Hispanics, African‐Americans, and non‐Hispanic whites. Am J Cardiol. 2016;117:76–83. doi: 10.1016/j.amjcard.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 25. Pfister R, Brägelmann J, Michels G, Wareham NJ, Luben R, Khaw K‐T. Performance of the CHARGE‐AF risk model for incident atrial fibrillation in the EPIC Norfolk cohort. Eur J Prev Cardiolog. 2015;22:932–939. doi: 10.1177/2047487314544045 [DOI] [PubMed] [Google Scholar]
- 26. Christophersen IE, Yin X, Larson MG, Lubitz SA, Magnani JW, McManus DD, Ellinor PT, Benjamin EJ. A comparison of the CHARGE–AF and the CHA2DS2‐VASc risk scores for prediction of atrial fibrillation in the Framingham Heart Study. Am Heart J. 2016;178:45–54. doi: 10.1016/j.ahj.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alonso A, Roetker NS, Soliman EZ, Chen LY, Greenland P, Heckbert SR. Prediction of atrial fibrillation in a racially diverse cohort: the Multi‐Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc. 2016;5:e003077. doi: 10.1161/JAHA.115.003077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Volgman AS, Bairey Merz CN, Benjamin EJ, Curtis AB, Fang MC, Lindley KJ, Pepine CJ, Vaseghi M, Waldo AL, Wenger NK, et al. Sex and race/ethnicity differences in atrial fibrillation. J Am Coll Cardiol. 2019;74:2812–2815. doi: 10.1016/j.jacc.2019.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chung MK, Refaat M, Shen W‐K, Kutyifa V, Cha Y‐M, Di Biase L, Baranchuk A, Lampert R, Natale A, Fisher J, et al. Atrial fibrillation: JACC council perspectives. J Am Coll Cardiol. 2020;75:1689–1713. doi: 10.1016/j.jacc.2020.02.025 [DOI] [PubMed] [Google Scholar]
- 30. Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet. 2007;370:1089–1100. doi: 10.1016/S0140-6736(07)61305-1 [DOI] [PubMed] [Google Scholar]
- 31. Albus C, Waller C, Fritzsche K, Gunold H, Haass M, Hamann B, Kindermann I, Köllner V, Leithäuser B, Marx N, et al. Significance of psychosocial factors in cardiology: update 2018: position paper of the German Cardiac Society. Clin Res Cardiol. 2019;108:1175–1196. doi: 10.1007/s00392-019-01488-w [DOI] [PubMed] [Google Scholar]
- 32. Garg PK, O'Neal WT, Diez‐Roux AV, Alonso A, Soliman EZ, Heckbert S. Negative affect and risk of atrial fibrillation: MESA. J Am Heart Assoc. 2019;8:e010603. doi: 10.1161/JAHA.118.010603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frasure‐Smith N, Lespérance F. Depression and other psychological risks following myocardial infarction. Arch Gen Psychiatry. 2003;60:627–636. doi: 10.1001/archpsyc.60.6.627 [DOI] [PubMed] [Google Scholar]
- 34. Clark CJ, Henderson KM, de Leon CFM, Guo H, Lunos S, Evans DA, Everson‐Rose SA. Latent constructs in psychosocial factors associated with cardiovascular disease: an examination by race and sex. Front Psychiatry. 2012;3:5. doi: 10.3389/fpsyt.2012.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jabson JM, Bowen D, Weinberg J, Kroenke C, Luo J, Messina C, Shumaker S, Tindle HA. Psychosocial clusters and their associations with well‐being and health: an empirical strategy for identifying psychosocial predictors most relevant to racially/ethnically diverse women's health. Clin Med Insights Womens Health. 2016;9:31–40. doi: 10.4137/CMWH.S34692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kop WJ. Chronic and acute psychological risk factors for clinical manifestations of coronary artery disease. Psychosom Med. 1999;61:476–487. doi: 10.1097/00006842-199907000-00012 [DOI] [PubMed] [Google Scholar]
- 37. Eisenmann ED, Rorabaugh BR, Zoladz PR. Acute stress decreases but chronic stress increases myocardial sensitivity to ischemic injury in rodents. Front Psychiatry. 2016;7:71. doi: 10.3389/fpsyt.2016.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kadish A, Jacobson J. Sleep patterns and arrhythmias: should this keep us awake at night? J Am Coll Cardiol. 2021;78:1208–1209. doi: 10.1016/j.jacc.2021.07.024 [DOI] [PubMed] [Google Scholar]
- 39. Chokesuwattanaskul R, Thongprayoon C, Sharma K, Congrete S, Tanawuttiwat T, Cheungpasitporn W. Associations of sleep quality with incident atrial fibrillation: a meta‐analysis. Intern Med J. 2018;48:964–972. doi: 10.1111/imj.13764 [DOI] [PubMed] [Google Scholar]
- 40. Lee H‐H, Chen Y‐C, Chen J‐J, Lo S‐H, Guo Y‐L, Hu H‐Y. Insomnia and the risk of atrial fibrillation: a population‐based cohort study. Acta Cardiol Sin. 2017;33:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li X, Zhou T, Ma H, Huang T, Gao X, Manson JE, Qi L. Healthy sleep patterns and risk of incident arrhythmias. J Am Coll Cardiol. 2021;78:1197–1207. doi: 10.1016/j.jacc.2021.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stein PK, Pu Y. Heart rate variability, sleep and sleep disorders. Sleep Med Rev. 2012;16:47–66. doi: 10.1016/j.smrv.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 43. Zaslavsky O, LaCroix AZ, Hale L, Tindle H, Shochat T. Longitudinal changes in insomnia status and incidence of physical, emotional, or mixed impairment in postmenopausal women participating in the Women's Health Initiative (WHI) study. Sleep Med. 2015;16:364–371. doi: 10.1016/j.sleep.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 44. Svensson T, Kitlinski M, Engström G, Melander O. Psychological stress and risk of incident atrial fibrillation in men and women with known atrial fibrillation genetic risk scores. Sci Rep. 2017;7:42613. doi: 10.1038/srep42613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kershaw KN, Brenes GA, Charles LE, Coday M, Daviglus ML, Denburg NL, Kroenke CH, Safford MM, Savla T, Tindle HA, et al. Associations of stressful life events and social strain with incident cardiovascular disease in the Women's Health Initiative. J Am Heart Assoc. 2014;3:e000687. doi: 10.1161/JAHA.113.000687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miao Jonasson J, Hendryx M, Shadyab AH, Kelley E, Johnson KC, Kroenke CH, Garcia L, Lawesson S, Santosa A, Sealy‐Jefferson S, et al. Social support, social network size, social strain, stressful life events, and coronary heart disease in women with type 2 diabetes: a cohort study based on the Women's Health Initiative. Diabetes Care. 2020;43:1759–1766. doi: 10.2337/dc19-2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mischel W, Shoda Y. A cognitive‐affective system theory of personality: reconceptualizing situations, dispositions, dynamics, and invariance in personality structure. Psychol Rev. 1995;102:246–268. doi: 10.1037/0033-295X.102.2.246 [DOI] [PubMed] [Google Scholar]
- 48. Barańczuk U. The five factor model of personality and social support: a meta‐analysis. J Res Pers. 2019;81:38–46. doi: 10.1016/j.jrp.2019.05.002 [DOI] [Google Scholar]
- 49. Garcia L, Follis S, Thomson CA, Breathett K, Cené CW, Jimenez M, Kooperberg C, Masaki K, Paskett ED, Pettinger M, et al. Taking action to advance the study of race and ethnicity: the Women's Health Initiative (WHI). Womens Midlife Health. 2022;8:1. doi: 10.1186/s40695-021-00071-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scheier MF, Carver CS. Optimism, coping, and health: assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4:219–247. doi: 10.1037/0278-6133.4.3.219 [DOI] [PubMed] [Google Scholar]
- 51. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-B [DOI] [PubMed] [Google Scholar]
- 52. Wang C, Lê‐Scherban F, Taylor J, Salmoirago‐Blotcher E, Allison M, Gefen D, Robinson L, Michael YL. Associations of job strain, stressful life events, and social strain with coronary heart disease in the Women's Health Initiative observational study. J Am Heart Assoc. 2021;10:e017780. doi: 10.1161/JAHA.120.017780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cook WW, Medley DM. Proposed hostility and pharisaic‐virtue scales for the MMPI. J Appl Psychol. 1954;38:414–418. doi: 10.1037/h0060667 [DOI] [Google Scholar]
- 54. Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB. The Cook‐Medley hostility scale: item content and ability to predict survival. Psychosom Med. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005 [DOI] [PubMed] [Google Scholar]
- 55. King LA, Emmons RA. Conflict over emotional expression: psychological and physical correlates. J Pers Soc Psychol. 1990;58:864–877. doi: 10.1037/0022-3514.58.5.864 [DOI] [PubMed] [Google Scholar]
- 56. Michael YL, Perrin N, Bowen D, Cochrane BB, Wisdom JP, Brzyski R, Ritenbaugh C. Expression and ambivalence over expression of negative emotion:psychometric analysis in the Women's Health Initiative. J Women Aging. 2005;17:5–18. doi: 10.1300/J074v17n01_02 [DOI] [PubMed] [Google Scholar]
- 57. Levine DW, Dailey ME, Rockhill B, Tipping D, Naughton MJ, Shumaker SA. Validation of the Women's Health Initiative insomnia rating scale in a multicenter controlled clinical trial. Psychosom Med. 2005;67:98–104. doi: 10.1097/01.psy.0000151743.58067.f0 [DOI] [PubMed] [Google Scholar]
- 58. Levine DW, Kaplan RM, Kripke DF, Bowen DJ, Naughton MJ, Shumaker SA. Factor structure and measurement invariance of the Women's Health Initiative insomnia rating scale. Psychol Assess. 2003;15:123–136. doi: 10.1037/1040-3590.15.2.123 [DOI] [PubMed] [Google Scholar]
- 59. Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Med Care. 1988;26:775–789. doi: 10.1097/00005650-198808000-00004 [DOI] [PubMed] [Google Scholar]
- 60. Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
References 50–60
Data Availability Statement
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the WHI at email: p&p@whi.org or website: https://www.whi.org/propose‐a‐paper.


