Abstract
Objectives
To describe selected baseline characteristics, continuation with baricitinib and disease activity over time in patients initiating treatment with baricitinib in a UK real-world rheumatology setting.
Methods
Baseline and follow-up data were analysed from baricitinib-treated patients newly recruited to the British Society for Rheumatology Biologics Registry–RA (BSRBR-RA) baricitinib cohort between 1 January 2018 and 31 March 2020. The primary objective was to evaluate continuation of baricitinib treatment in patients with at least one follow-up. Analyses were performed using the full baricitinib cohort, overall and by patient subgroup: biologic DMARD (bDMARD)/targeted synthetic (ts)DMARD-naive vs -experienced, baricitinib 4 vs 2 mg, age ≥65 vs <65 years, monotherapy vs combination therapy and male vs female.
Results
At baseline, the study cohort (n = 561) was 76.5% female, mean age 60.0 years, had longstanding (mean 13.1 years) and severe RA, and 54.0% had previously received a bDMARD/tsDMARD. Of 265 and 110 patients completing the 6- and 12-month follow-ups with available data, 77.7 and 69.1% remained on baricitinib at each time, respectively. In all Kaplan–Meier analyses, >60% of patients remained on baricitinib at 540 days. Continuation of baricitinib therapy differed between some subgroup pairs (bDMARD/tsDMARD naive/experienced, baricitinib 2 mg/4 mg). Disease activity was lower at both follow-ups than at baseline, overall and in all subgroups.
Conclusion
In the early years of real-world baricitinib use in the UK, a high proportion of patients continued with treatment at both 6 and 12 months, at which times disease activity was lower than at baseline.
Keywords: baricitinib, Janus kinase inhibitor, observational study, RA, real-world, registry
Rheumatology key messages.
Most patients initiating baricitinib were female, had longstanding and severe rheumatoid arthritis and were bDMARD-experienced.
A high proportion of patients continued treatment at both 6 (77.7%) and 12 (69.1%) months.
Disease activity was lower overall and by subgroup at 6 and 12 months vs baseline.
Introduction
The targeted synthetic DMARD (tsDMARD) baricitinib is a once-daily oral selective Janus kinase (JAK)-1 and JAK2 inhibitor that was approved in Europe in 2017 for the treatment of moderate-to-severe active RA at a standard dose of 4 mg once daily alone or in combination with methotrexate in adults who have responded inadequately to or are intolerant to one or more DMARDs; a dose of 2 mg once daily is appropriate for selected patients and may be considered for patients who have achieved sustained control of disease activity with 4 mg once daily and are eligible for dose tapering [1].
In 2017, the UK National Institute for Health and Care Excellence included baricitinib in its pathways and recommendations for the treatment of RA [2]. Specifically, baricitinib is recommended for use with methotrexate or, if methotrexate is contraindicated/not tolerated, as monotherapy for patients with a DAS for 28-joint count (DAS28) of >5.1 and either an inadequate response to a combination of conventional synthetic DMARDS (csDMARDs) or an inadequate response or contraindication/intolerance to other DMARDs, including rituximab. Treatment with baricitinib can only be continued if there is a moderate response, measured using European Alliance of Associations for Rheumatology (EULAR) criteria, at 6 months after starting therapy; after an initial response, treatment should be withdrawn if at least a moderate EULAR response is not maintained. In 2020, baricitinib was additionally approved for the treatment of moderate-to-severe atopic dermatitis.
The British Society for Rheumatology Biologics Registry–Rheumatoid Arthritis (BSRBR-RA) was established in 2001 to study the safety of biologic DMARDs (bDMARDs) used in routine clinical care in patients with RA in the UK and has regularly been expanded to incorporate new treatments, including tsDMARDS. Patients enrolled in the registry are followed up at 6-monthly intervals for the first 3 years and annually thereafter.
The aim of this study was therefore to report real-world experience of early use of baricitinib; this analysis describes selected baseline characteristics, continuation with baricitinib and disease activity over time in patients enrolled in the BSRBR-RA registry initiating treatment with baricitinib.
Methods
Study design
This observational study analysed baseline and follow-up data, when available, from patients receiving baricitinib who were recruited to the BSRBR-RA registry. The current analysis builds on a previous study performed using an earlier baricitinib dataset from the registry [3, 4].
Eligible baricitinib-treated patients were registered to the BSRBR-RA baricitinib cohort (either newly registered to the BSRBR-RA or re-registered after switching from another product in the registry). Characteristics of patients at the time of baricitinib initiation were submitted no later than 6 months after the start of therapy and included demographics and DAS28. Follow-up forms capturing clinical data recorded from regular clinic visits were completed every 6 months from the date of baricitinib initiation to record changes in therapy and post-baseline outcomes in the BSRBR-RA registry. Because the follow-ups were not scheduled study visits, it is possible that follow-up information is missing for some patients.
The current analyses include data from baseline and the 6-month (follow-up 1) and 12-month (follow-up 2) follow-ups of patients starting baricitinib; these data were obtained from a routine annual dataset shared with Eli Lilly and Company as part of a contractual agreement between the British Society for Rheumatology (BSR) and Eli Lilly and Company. The analyses were independent of the BSR and the academic team who run the BSRBR-RA at the University of Manchester, who had no involvement in the planning or analyses of data or preparation of the manuscript. No calculations of sample size were performed as the study sample was determined by the number of eligible patients enrolled in the BSRBR-RA registry at the time of database lock on 31 March 2020.
This BSRBR-RA study was approved by the North West Multicentre Research Ethics Committee (MREC) on 1 December 2000, reference 00/8/053. Written informed consent was obtained from the subjects (or their legally authorized representative) at entry to the BSRBR-RA. The original consent to be involved in the BSRBR-RA observational study covers the current analyses. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with good pharmacoepidemiology practices and applicable laws and regulations of the UK.
Population
Data from patients initiating baricitinib and registered in the BSRBR-RA baricitinib cohort between 1 January 2018 and 31 March 2020 were included in this analysis. To be eligible for the current analysis, patients were required to be aged ≥18 years at the time of baricitinib initiation and to have received at least one dose of baricitinib 2 or 4 mg for the treatment of RA [fulfilled American College of Rheumatology (ACR) classification criteria at the time of registration or were diagnosed with RA by a consultant rheumatologist]. Other baricitinib-specific UK treatment eligibility requirements were assumed to have been met.
Analyses
Primary objective
The primary objective of this analysis was to evaluate continuation of baricitinib treatment. The drug was considered to be discontinued when a stop date was recorded in the BSRBR-RA, with no new start date recorded within the following 28 days. The probability of patients continuing baricitinib over time was determined using Kaplan–Meier analysis. Kaplan–Meier plots up to 540 days were created using all available data on baricitinib start and stop dates, with censoring at date of last follow-up recorded before 31 March 2020 or date of death, whichever was earliest. Transition to an off-label dose of baricitinib (not 2 or 4 mg) was considered as discontinuation; however, treatment interruptions of ≤28 days were permitted. Baricitinib continuation was also summarized as the number (percentage) of patients remaining on baricitinib at follow-up clinic visits.
Secondary objectives
Patient demographics and characteristics, as well as disease characteristics, at the time of baricitinib initiation were reported descriptively. Findings were recorded as number (percentage) for categorical variables and mean (s.d.) and median [interquartile range (IQR)] for continuous variables.
Reasons for baricitinib discontinuation were recorded when available and were classified as lack of efficacy, adverse event (including death) or other (all other reasons). The study dataset did not include data on specific adverse events.
At follow-up, mean (s.d.) and median (IQR) DAS28 using ESR (DAS28-ESR) and the number (percentage) of patients in each DAS28-ESR category [remission <2.6, low disease activity (LDA) >2.6 to ≤3.2, moderate disease activity (MDA) >3.2 to ≤5.1 and high disease activity (HDA) >5.1] were reported for those completing follow-up with available DAS28-ESR data and who had not discontinued baricitinib prior to the follow-up.
Data analysis
Analyses were performed using the full baricitinib cohort and five patient subgroups: (i) monotherapy vs combination therapy; (ii) bDMARD/tsDMARD-naive (prior csDMARD only) vs bDMARD/tsDMARD-experienced (i.e. prior exposure to at least one bDMARD/tsDMARD: yes vs no); (iii) baricitinib 2 vs 4 mg; (iv) male vs female; and (v) age ≥65 vs <65 years. For the assessment of baricitinib continuation and DAS28-ESR post-baseline, patients were required to have at least one follow-up report after the report of baricitinib initiation; for other objectives, all patients were eligible. All analyses were descriptive, and no statistical testing was performed.
If information on age and/or the dose of baricitinib was missing at baseline, patients were excluded from the analysis. For calculations involving dates, the day and month of birth and diagnosis of RA was assumed to be 30 June for all patients (only year was captured in the database) and date of death was assumed to be the 15th of the month (only month and year were captured in the database). If the dates of the 6- or 12-month follow-up were missing, they were imputed for the purposes of censoring in the Kaplan–Meier analyses as start date plus 6 or 12 months, respectively.
Results
Baseline data were available from 561 patients in the baricitinib cohort who met study eligibility requirements; of these, 272 had completed at least one follow-up.
Population and disease characteristics
The overall baricitinib population had a mean age of 60.0 years and mean RA duration of 13.1 years (Table 1). More than half (60.6%) were taking baricitinib with a csDMARD and 54.0% had received prior bDMARD/tsDMARD therapy. The majority of patients were female (76.5%), overweight (BMI ≥25.0 kg/m2 [5]) and treated with baricitinib 4 mg. More than half of patients were current (13.8%) or past (39.0%) smokers. Mean (s.d.) and median (IQR) baseline DAS28-ESR values were 5.7 (1.2) and 5.7 (5.2–6.4), respectively. Overall, 77.6% of patients had HDA, 18.6% had MDA, 1.4% had LDA and 2.3% were in remission.
Table 1.
Baseline characteristics of 561 patients enrolled in the BSRBR-RA baricitinib cohort
| Characteristics | BSRBR-RA baricitinib cohort |
||
|---|---|---|---|
| n with non-missing data | Mean (s.d.)/n (%) | Median (IQR) | |
| Age (years) | 561 | 60.0 (12.0) | 61.1 (53.0–68.8) |
| Sex (female) | 561 | 429 (76.5) | |
| BMI (kg/m2) | 296 | 28.1 (6.4) | 27.0 (23.4–32.0) |
| BMI category | 296 | ||
| Underweight (<18.5 kg/m2) | 7 (2.4) | ||
| Normal (18.5–24.9 kg/m2) | 94 (31.8) | ||
| Overweight (≥25.0 kg/m2) | 195 (65.9) | ||
| Rheumatoid factor positive (yes) | 432 | 289 (66.9) | |
| Smoking category | 413 | ||
| Current | 57 (13.8) | ||
| Ex-smoker | 161 (39.0) | ||
| Never smoked | 195 (47.2) | ||
| Disease duration (years) | 533 | 13.1 (10.3) | 11.0 (5.0–19.0) |
| Baseline DAS28-ESR | 559 | 5.7 (1.2) | 5.7 (5.2–6.4) |
| DAS28-ESR category | 559 | ||
| Remission | 13 (2.3) | ||
| Low disease activity | 8 (1.4) | ||
| Moderate disease activity | 104 (18.6) | ||
| High disease activity | 434 (77.6) | ||
| Baseline ESR | 255 | 29.7 (24.5) | 24.0 (12.0–40.0) |
| Baseline CRP | 269 | 17.8 (23.9) | 24.0 (4.0–22.0) |
| Baseline HAQ-DI | 119 | 1.7 (0.7) | 1.9 (1.4–2.3) |
| History of | 561 | ||
| Asthma | 75 (13.4) | ||
| COPD | 41 (7.3) | ||
| Respiratory disease (asthma/COPD) | 107 (19.1) | ||
| Angina | 14 (2.5) | ||
| MI | 22 (3.9) | ||
| Stroke | 10 (1.8) | ||
| CVD (angina or MI or stroke) | 38 (6.8) | ||
| Diabetes | 45 (8.0) | ||
| Previously treated with biologic (yes) | 561 | 303 (54.0) | |
| Baricitinib dosing | 561 | ||
| Dose prescribed 2 mg | 83 (14.8) | ||
| Dose prescribed 4 mg | 478 (85.2) | ||
| Use with csDMARD (yes) | 340 (60.6) | ||
| Use with methotrexate (yes) | 237 (42.3) | ||
| Use with steroid (yes) | 142 (25.3) | ||
DAS28-ESR categories: remission (DAS28-ESR <2.6), low disease activity (>2.6 to ≤3.2), moderate disease activity (>3.2 to ≤5.1) and severe/high disease activity (>5.1).
BSRBR-RA: British Society for Rheumatology Biologics Registry–RA; COPD: chronic obstructive pulmonary disease (chronic bronchitis/emphysema); csDMARD: conventional systemic DMARD; CVD: cardiovascular disease; DAS28-ESR: DAS for 28-joint count using ESR; HAQ-DI: HAQ Disability Index; IQR: interquartile range; MI: myocardial infarction.
Numbers of patients were unevenly distributed in the different subgroups considered, as a result of baseline characteristics of the study population, with more patients in the combination therapy vs monotherapy, baricitinib 4 vs 2 mg, female vs male, and younger vs older subgroups (Supplementary Tables S1–S5, available at Rheumatology online). Review of baseline information between each subgroup pairing showed some differences in patient characteristics; those presenting with an imbalance between the subgroup pairings are identified in Supplementary Tables S1–S5, available at Rheumatology online, by use of italics.
Continuation and discontinuation of baricitinib
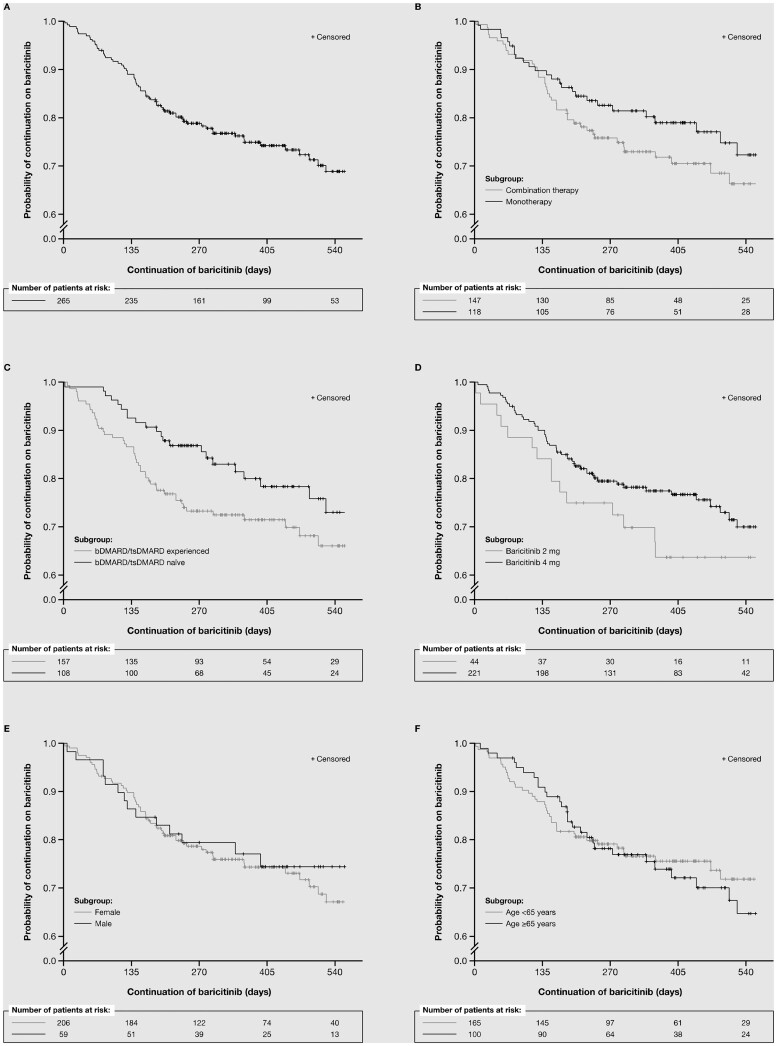
Kaplan–Meier plots of baricitinib continuation, overall and by subgroup, showed that >60% of patients remained on treatment at 540 days (Fig. 1). Of 265 patients completing the 6-month follow-up, 206 (77.7%) remained on baricitinib at this time. Of 110 patients completing the 12-month follow-up, 76 (69.1%) remained on baricitinib at this time (Table 2). Patients appeared more likely to have continued baricitinib therapy at both the 6- and 12-month follow-ups if they were in the following subgroups: bDMARD/tsDMARD-naïve vs -experienced and baricitinib 4 vs 2 mg. At the 6-month follow-up, there was little difference in baricitinib continuation between the monotherapy vs combination therapy, male vs female, and age ≥65 vs <65 years subgroups. However, at the 12-month follow-up, patients in the monotherapy, male and younger age subgroups appeared more likely to remain on therapy than those in the combination therapy, female and older age subgroups, respectively, although the numbers of patients in each subgroup at this time were small.
Figure 1.
Kaplan–Meier plots of baricitinib survival over time for the overall cohort and patient subgroups. (A) Overall population. (B) Therapy subgroups (monotherapy vs combination therapy). (C) Previous therapy subgroups [bDMARD/tsDMARD naive (prior csDMARD only) vs bDMARD/tsDMARD experienced]. (D) Baricitinib dose subgroups (2 vs 4 mg). (E) Sex subgroups (male vs female). (F) Age subgroups (≥65 vs <65 years). bDMARD: biologic DMARD; csDMARD: conventional systemic DMARD; pts: patients; tsDMARD: targeted synthetic DMARD
Table 2.
Proportion of patients continuing baricitinib at follow-up, for the overall cohort and patient subgroups
| Population and subgroups | 6-month follow-up |
12-month follow-up |
||
|---|---|---|---|---|
| N | n (%) | N | n (%) | |
| Overall population | 265 | 206 (77.7) | 110 | 76 (69.1) |
| Therapy subgroups | ||||
| Monotherapy | 118 | 95 (80.5) | 47 | 36 (76.6) |
| Combination therapy | 147 | 111 (75.5) | 63 | 40 (63.5) |
| Previous therapy subgroups | ||||
| bDMARD/tsDMARD naïve (prior csDMARD only) | 108 | 91 (84.3) | 49 | 36 (73.5) |
| bDMARD/tsDMARD experienced | 157 | 115 (73.3) | 61 | 40 (65.6) |
| Baricitinib dose subgroups | ||||
| 2 mg | 44 | 31 (70.5) | 19 | 10 (52.6) |
| 4 mg | 221 | 175 (79.2) | 91 | 66 (72.5) |
| Sex subgroups | ||||
| Male | 59 | 47 (79.7) | 28 | 21 (75.0) |
| Female | 206 | 159 (77.2) | 82 | 55 (67.1) |
| Age subgroups | ||||
| ≥65 years | 100 | 77 (77.0) | 46 | 29 (63.0) |
| <65 years | 165 | 129 (78.2) | 64 | 47 (73.4) |
For patients completing at least one follow-up.
bDMARD: biologic DMARD; csDMARD: conventional systemic DMARD; N: number of patients with follow-up data; tsDMARD: targeted synthetic DMARD.
The most common reason for baricitinib discontinuation at both follow-ups was adverse events (about 63% of both the 57 and 32 patients who discontinued and had a reason recorded at the 6- and 12-month follow-up, respectively; Table 3).
Table 3.
Reasons for discontinuation of baricitinib in the BSRBR-RA
| Reason for discontinuation | n | % |
|---|---|---|
| 6-month follow-up | ||
| Lack of efficacy | 14 | 24.6 |
| Adverse events | 36 | 63.2 |
| Other | 7 | 12.3 |
| 12-month follow-up | ||
| Lack of efficacy | 8 | 25.0 |
| Adverse events | 20 | 62.5 |
| Other | 4 | 12.5 |
A total of 59 patients with at least one recorded follow-up discontinued baricitinib by the 6-month follow-up and 34 patients with at least one recorded follow-up discontinued baricitinib by the 12-month follow-up; two patients had missing data at the 6-month follow-up and two patients had missing data at the 6-month follow-up.
n: number of patients with reported reason.
Severity of rheumatoid arthritis
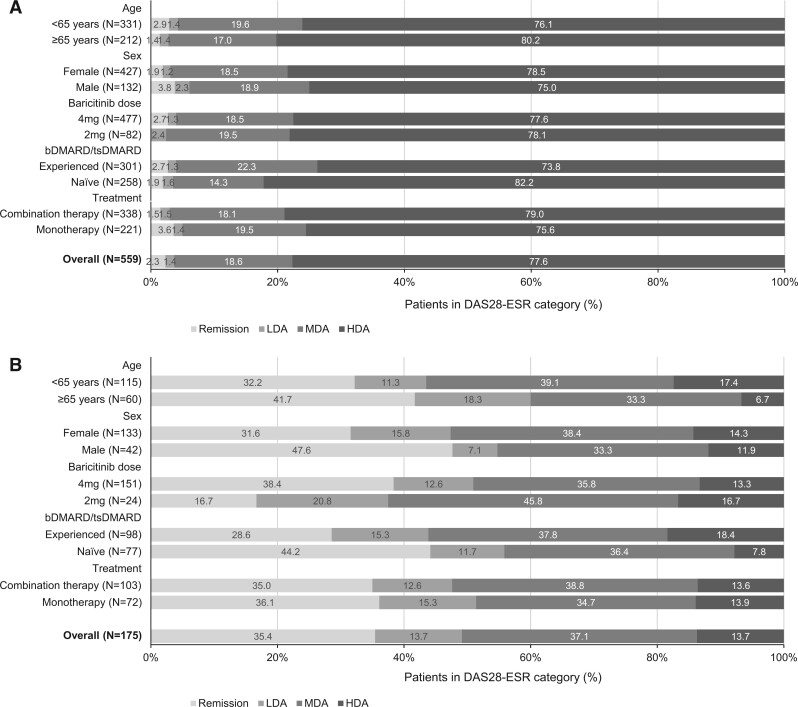
Overall, mean (s.d.) and median (IQR) DAS28-ESR scores were lower at the 6-month follow-up [3.3 (1.5) and 3.2 (2.2–4.2), respectively] than at baseline; this pattern was observed in all subgroups (Table 4). Examination of subgroups suggested that, at the 6-month follow-up, mean and median DAS28-ESR scores were lower in bDMARD/tsDMARD-naive vs -experienced patients, patients receiving baricitinib 4 vs 2 mg, and those aged ≥65 vs <65 years (Table 4). The proportions of patients in each DAS28-ESR category supported these findings: overall, 35.4% of patients were in remission, 13.7% had LDA, 37.1% had MDA and 13.7% had HDA (Fig. 2). Notably, at the 6-month follow-up, 44.2 and 28.6% of bDMARD/tsDMARD-naive and -experienced patients, respectively, and 38.4 and 16.7% of patients in the 4 and 2 mg subgroups, respectively, were in remission. Remission rates also appeared to differ according to age and sex at the 6-month follow-up, with males and those aged ≥65 years achieving higher rates than females and those aged <65 years, respectively. There was little difference in DAS28-ESR levels at the 6-month follow-up between the monotherapy and combination therapy subgroups (Table 4, Fig. 2).
Table 4.
DAS28-ESR at baseline and the 6-month follow-up after baricitinib initiation, for overall cohort and patient subgroups
| Population and subgroups | DAS28-ESR at baseline |
DAS28-ESR at the 6-month follow-up |
||||
|---|---|---|---|---|---|---|
| N | Mean (s.d.) | Median (IQR) | N | Mean (s.d.) | Median (IQR) | |
| Overall population | 559 | 5.7 (1.2) | 5.7 (5.2–6.4) | 175 | 3.3 (1.5) | 3.2 (2.2–4.2) |
| Therapy subgroups | ||||||
| Monotherapy | 221 | 5.6 (1.3) | 5.7 (5.1–6.4) | 72 | 3.3 (1.4) | 3.2 (2.2–4.0) |
| Combination therapy | 338 | 5.7 (1.1) | 5.7 (5.2–6.4) | 103 | 3.4 (1.5) | 3.3 (2.2–4.3) |
| Previous therapy subgroups | ||||||
| bDMARD/tsDMARD naïve (prior csDMARD only) | 258 | 5.6 (1.1) | 5.6 (5.2–6.2) | 77 | 3.0 (1.4) | 2.7 (2.0–3.7) |
| bDMARD/tsDMARD experienced | 301 | 5.7 (1.2) | 5.7 (5.1–6.5) | 98 | 3.6 (1.4) | 3.4 (2.5–4.5) |
| Baricitinib dose subgroups | ||||||
| 2 mg | 82 | 5.8 (1.1) | 5.8 (5.2–6.4) | 24 | 4.0 (1.5) | 3.8 (3.0–4.8) |
| 4 mg | 477 | 5.6 (1.2) | 5.7 (5.2–6.4) | 151 | 3.3 (1.4) | 3.2 (2.1–4.0) |
| Sex subgroups | ||||||
| Male | 132 | 5.5 (1.2) | 5.5 (5.1–6.3) | 42 | 3.1 (1.5) | 3.0 (1.9–4.0) |
| Female | 427 | 5.7 (1.1) | 5.8 (5.2–6.4) | 133 | 3.4 (1.4) | 3.3 (2.4–4.3) |
| Age subgroups | ||||||
| ≥65 years | 212 | 5.8 (1.1) | 5.7 (5.2–6.5) | 60 | 3.1 (1.3) | 2.9 (2.1–3.9) |
| <65 years | 347 | 5.6 (1.2) | 5.6 (5.1–6.3) | 115 | 3.5 (1.5) | 3.4 (2.2–4.4) |
Analyses performed in patients completing the 6-month follow-up with available DAS28-ESR data and who had not discontinued baricitinib prior to the follow-up.
bDMARD: biologic DMARD; csDMARD: conventional systemic DMARD; DAS28-ESR: DAS for 28-joint count using ESR; IQR: interquartile range; N: number of patients with follow-up data; tsDMARD: targeted synthetic DMARD.
Figure 2.
DAS28-ESR at (A) baseline and (B) the 6-month follow-up after baricitinib initiation. For the overall cohort and patient subgroups (patients completing the 6-month follow-up with available DAS28-ESR data and who had not discontinued baricitinib prior to the follow-up) by DAS28-ESR category: remission (DAS28-ESR <2.6), LDA (>2.6 to ≤3.2), MDA (>3.2 to ≤5.1) and HDA (>5.1). bDMARD: biologic DMARD; csDMARD: conventional systemic DMARD; DAS28-ESR: DAS for 28-joint count using ESR; HDA: high disease activity; LDA: low disease activity; MDA: moderate disease activity; N: number of patients with follow-up data; tsDMARD: targeted synthetic DMARD
Data were available for a total of 52 evaluable patients at the 12-month follow-up; numbers in each subgroup were too small to provide meaningful information and are therefore not presented. For the overall population, mean (s.d.) and median (IQR) DAS28-ESR at the 12-month follow-up were 3.3 (1.3) and 3.3 (2.3–4.0), respectively, and 32.7% of patients were in remission, 13.5% had LDA, 44.2% had MDA and 9.6% had HDA.
Discussion
This observational study used real-world data on the treatment of RA with baricitinib, collected prospectively in the BSRBR-RA registry. These analyses have provided important information about the characteristics and outcomes of patients with RA initiating baricitinib in the UK. The current study covers the early baricitinib post-launch period in the UK and predates the major disruption of UK healthcare services due to the COVID-19 pandemic. The proportions of patients continuing baricitinib at the 6- and 12-month follow-ups were high at 77.7 and 69.1%, respectively. Kaplan–Meier plots indicated that, overall and in all subgroups, >60% of patients remained on therapy at 540 days (∼18 months). These findings for an oral therapy are encouraging and appear broadly similar to continuation rates at 1 year reported for a first tumour necrosis factor-α inhibitor or tocilizumab [6, 7] but lower than that reported for rituximab [8] in the BSRBR-RA.
In general, the study cohort of patients initiating treatment with baricitinib was predominantly female, with a mean age of 60.0 years, and had longstanding, pre-treated (with a bDMARD/tsDMARD) RA and HDA. Patients had notable functional disability (mean Health Assessment Questionnaire Disability Index 1.7). Characteristics of the current baricitinib BSRBR-RA cohort were generally similar to those described for other BSRBR-RA bDMARD/tsDMARD cohorts [7–11], reflecting previous access to bDMARD or tsDMARD therapy in the UK at the time baricitinib therapy was initiated in most patients included in this analysis (where treatment with two csDMARDs must have failed and patients must have HDA to be eligible for bDMARD or tsDMARD therapy). A small proportion of patients had LDA or remission (3.7% of the total cohort) at initiation of baricitinib. The reason for this is not known, but we can speculate that this was because patients switched to baricitinib from another treatment as a result of tolerability issues.
Generally, few notable differences in baseline characteristics were identified across the patient subgroups considered, although no formal statistical testing was performed. However, it is worth noting that patients receiving the lower dose of baricitinib (2 mg) were older, had greater functional disability, and were more likely to have a history of asthma and previous bDMARD therapy than those receiving baricitinib 4 mg. This may suggest that clinicians use a lower dose of the drug for patients considered to be older and frailer. In addition, patients with asthma are at increased risk of infection [12], which may have influenced physicians’ dosing decisions regarding the use of baricitinib 2 mg. Patients in the bDMARD/tsDMARD-naive subgroup were more likely to be male and have a shorter RA duration, severe RA and higher C-reactive protein (CRP) levels than the bDMARD/tsDMARD-experienced subgroup, suggesting that clinicians are prescribing baricitinib before bDMARDs for selected patients, including males and patients with more severe RA. Patients receiving baricitinib as monotherapy were more likely to have received previous bDMARD therapy, and a greater proportion were receiving concomitant steroid therapy than those in the combination therapy subgroup, suggesting that these former patients may have had unsatisfactory outcomes with or contraindications to previous csDMARD combinations.
The high proportion of patients continuing baricitinib therapy at 6 months (77.7%) is consistent with baricitinib data from a post-marketing surveillance study in Japan (74.4%) [13], a Japanese multicentre biologics registry (the Tsurumai Biologics Communication Registry; 86.5%) [14], the Swiss Clinical Quality Management-RA (SCQM-RA) register (>75%) [15] and the prospective observational study RA-BE-REAL (81.2%) [16]. The most common reason for discontinuation, when one was provided, was adverse events (a limitation of this study is that further details of adverse events were not available for analysis). In contrast, the most common reason for stopping baricitinib in other real-world studies was lack of efficacy [14, 17].
When baricitinib continuation was evaluated by subgroup, it appeared that a smaller proportion of patients continued treatment in the bDMARD/tsDMARD-experienced vs -naive subgroup, and the 2 vs 4 mg subgroup at the 6- and 12-month follow-ups, and the combination therapy vs monotherapy, female vs male and older vs younger age subgroups at the 12-month follow-up. Similarly, in the SCQM-RA register, combination therapy was associated with reduced continuation on baricitinib, but line of therapy was not [15]. In the Swiss register, the percentage of patients continuing baricitinib at 12 months (>75%) was similar to that in the current analysis (69.1%) and significantly higher than that for patients continuing tumour necrosis factor-α inhibitor therapy (both bDMARD-naive and -experienced) [18]. Analysis of other BSRBR-RA bDMARD/tsDMARD cohorts found that, although bDMARD/tsDMARD-naive patients were more likely to continue with rituximab or tocilizumab than bDMARD/tsDMARD-experienced patients, multivariable analyses or propensity score adjustment to remove confounders no longer showed these differences [7, 8]. At the 6-month follow-up, baricitinib continuation was similar in both age subgroups, and DAS28-ESR scores were actually lower in older than in younger patients, suggesting that age does not need to be a key consideration when selecting patients who may benefit from baricitinib. At the 12-month follow-up, >60% of patients in both age groups remained on baricitinib therapy.
We found that a substantial proportion of patients with RA were receiving baricitinib as monotherapy in the BSRBR-RA cohort (29.8% of the bDMARD/tsDMARD-naive population and 39.4% of the overall population). Data from the 6-month follow-up indicated that disease severity was similar in the baricitinib monotherapy and combination therapy subgroups. Mean DAS28-ESR at the 6-month follow-up was lower than at baseline in the overall population and all subgroups, and the proportions of patients in remission or with LDA at the 6-month follow-up were higher than at baseline. Patients providing data for the 12-month follow-up represent the earliest treated patients who may have had different characteristics to those of patients providing data at baseline and the 6-month follow-up. Nevertheless, at 12 months, mean DAS28-ESR remained low, and the proportions of patients in remission or with LDA remained elevated compared with baseline.
When specific subgroups were considered, sufficient data were available for the 6-month follow-up only. At this time, patients in the bDMARD/tsDMARD-naive and 4 mg subgroups had lower disease activity than the bDMARD/tsDMARD-experienced and 2 mg subgroups, respectively. Analysis of another BSRBR-RA bDMARD/tsDMARD cohort, in which change from baseline in disease activity was evaluated, found a greater difference between baseline and 6-month DAS28 scores in bDMARD/tsDMARD-experienced than -naive patients receiving rituximab therapy; however, adjustment analyses removed the difference [10]. In contrast, multivariable analysis of patients receiving baricitinib enrolled in the Japanese biologics registry revealed that no previous tsDMARD therapy and a lower DAS28-CRP score at baseline, but not concomitant methotrexate therapy, were independently associated with achievement of LDA [14].
Interpretation of the findings of our study must take into consideration the small numbers of patients providing follow-up data for some subgroups, particularly the 2 mg subgroup and all subgroups at the 12-month follow-up (only 110 patients overall completed the second follow-up). Subgroup findings at the 12-month follow-up should therefore be considered exploratory and be treated with caution. However, baricitinib 4 mg has been shown to have greater efficacy than the 2 mg dose [19], with similar tolerability [19–21], potentially explaining the higher rate of continuation and lower follow-up DAS28-ESR with the higher dose. Another potential contributor to these between-dose differences is the observed differences in baseline characteristics for each subgroup pairing, as already discussed.
The treatment of RA has been transformed by the availability of bDMARDs/tsDMARDs and treat-to-target strategies; however, the currently evolving therapeutic options necessitate high vigilance and carefully conducted studies to assess safety, efficacy and effectiveness [22]. National biologic registers provide valuable information on real-world effectiveness and safety beyond those observed in clinical trials and fill an important gap in the literature, complementing clinical trial data. For example, in common with many registry populations [22], our population differed in some respects from those of clinical trials of baricitinib [19–21, 23, 24].
Nonetheless, all registry data have some limitations, including that allocation of patients to treatment is not randomized, data are often missing, and the type and quality of data collected can vary across registries [22]. Limitations of the current analyses include that outcomes over time could only be reported in patients who had at least one recorded follow-up; although 561 patients started therapy with baricitinib in our study population, only 265 had a recorded first follow-up in the registry. Thus, many of the subgroups considered included only small patient numbers, preventing robust conclusions from being drawn. Additionally, another subgroup analysis was planned [overweight/obese (BMI ≥25.0 kg/m2) vs underweight/normal weight (BMI <25.0 kg/m2) [5]], but the numbers of patients providing BMI data were insufficient to allow this. Also, there was notable variability in some baseline characteristics between subgroups. In addition, our continuation estimates may be conservative as we employed the strict requirement that only gaps in baricitinib therapy of ≤28 days were allowed. Finally, the study period did not include the time when healthcare provision in the UK was disrupted by the COVID-19 pandemic. Future research might explore the effect of the pandemic on continuation with therapy and DAS28-ESR outcomes; however, missing follow-up data due to cessation of research activities during this period could provide a challenge for data analysis. In addition, it will be interesting to ascertain whether smoking status affects ongoing outcomes in the BSRBR-RA baricitinib cohort.
Conclusion
In the early years of real-world baricitinib use in the UK, a high proportion of patients continued with treatment at both 6 and 12 months, and disease activity was lower at these times than at baseline. It is notable that baricitinib performed well as monotherapy, providing a therapeutic option for patients unable to tolerate csDMARDs.
Supplementary Material
Contributor Information
Christopher J Edwards, NIHR Southampton Clinical Research Facility, University Hospital Southampton, Southampton, UK.
Julie Mount, Eli Lilly and Company Limited, Basingstoke, Hampshire, UK.
Alexandra Meeks, Eli Lilly and Company, Indianapolis, IN, USA.
Tania Gulati, Eli Lilly and Company (India) Pvt. Ltd, Gurgaon, Haryana, India.
Liliana Zaremba-Pechmann, HaaPACS GmbH, Schriesheim, Germany.
Mohamed Sheesh, Eli Lilly and Company Limited, Basingstoke, Hampshire, UK.
Esbjörn Larsson, Eli Lilly Sweden AB, Solna, Sweden.
Elaine Dennison, Faculty of Medicine, University of Southampton, Southampton, UK.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
The data underlying this article were provided to Lilly by the British Society for Rheumatology under licence. Data will be shared upon reasonable request to the corresponding author with the additional permission of the British Society for Rheumatology.
Funding
This work was supported by Eli Lilly and Company. Medical writing services were provided by Caroline Spencer and Dr Sue Chambers (Rx Communications, Mold, UK), funded by Eli Lilly and Company.
Disclosure statement: C.J.E. has received fees from Abbvie, Astra Zeneca, Fresenius Kabi, Gilead, Galapagos, GSK, Janssen, Eli Lilly and Company, Pfizer, Roche, Sanofi for advisory boards, speakers bureau and research support. J.M., M.S., T.G., A.M. and E.L. are employees of Eli Lilly and Company. J.M., M.S., T.G. and E.L. are shareholders of Eli Lilly and Company. L.Z.-P. is a consultant from HaaPACS. E.D. has received honoraria and speaker fees from UCB, Pfizer, Viatris and Eli Lilly and Company.
References
- 1. Lilly. Olumiant summary of product characteristics. 2017. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant (14 October 2021, date last accessed).
- 2. National Institute for Health and Care Excellence. Baricitinib for moderate to severe rheumatoid arthritis. Technology appraisal guidance [TA466]. 2017. https://www.nice.org.uk/guidance/ta466/chapter/1-Recommendations (17 October 2021, date last accessed).
- 3. Edwards CJ, Mount JE, Meeks AC. et al. PMS40 starting treatment with baricitinib: characteristics of patients and status at first follow-up in the BSRBR-RA registry. Value Health 2020;23:S599–600. [Google Scholar]
- 4. Edwards CJ, Mount JE, Meeks AC. et al. O33 Baricitinib in the BSRBR-RA registry: characteristics and status of patients at first follow-up. In: Proceedings of the British Society for Rheumatology Annual Conference, 26–28 April 2021. Rheumatology 2021;60(Suppl 1):keab246.032.
- 5. World Health Organization. Body mass index – BMI. 2021. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (24 November 2021, date last accessed).
- 6. Soliman MM, Ashcroft DM, Watson KD. et al. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2011;70:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kihara M, Davies R, Kearsley-Fleet L. et al. Use and effectiveness of tocilizumab among patients with rheumatoid arthritis: an observational study from the British Society for Rheumatology Biologics Register for rheumatoid arthritis. Clin Rheumatol 2017;36:241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oldroyd AGS, Symmons DPM, Sergeant JC. et al. Long-term persistence with rituximab in patients with rheumatoid arthritis. Rheumatology 2018;57:1089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamann PD, Pauling JD, McHugh N, Hyrich K, Shaddick G; BSRBR-RA Contributors Group. Early response to anti-TNF predicts long-term outcomes including sustained remission: an analysis of the BSRBR-RA. Rheumatology 2020;59:1709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soliman MM, Hyrich KL, Lunt M. et al. Effectiveness of rituximab in patients with rheumatoid arthritis: observational study from the British Society for Rheumatology Biologics Register. J Rheumatol 2012;39:240–6. [DOI] [PubMed] [Google Scholar]
- 11. Kearsley-Fleet L, Davies R, De Cock D. et al. Biologic refractory disease in rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2018;77:1405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kisiel MA, Zhou X, Björnsson E. et al. The risk of respiratory tract infections and antibiotic use in a general population and among people with asthma. ERJ Open Res 2021;7:00429–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujii T, Atsumi T, Okamoto N. et al. AB0249 Safety of baricitinib in Japanese patients with rheumatoid arthritis (RA): the 2020 interim report from all-case post marketing surveillance in clinical practice. In: Proceedings of the European Congress of Rheumatology, 2–5 June 2021, Virtual. Ann Rheum Dis 2021;80:1150. [Google Scholar]
- 14. Takahashi N, Asai S, Kobayakawa T. et al. Predictors for clinical effectiveness of baricitinib in rheumatoid arthritis patients in routine clinical practice: data from a Japanese multicenter registry. Sci Rep 2020;10:21907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilbert B, Lauper K, Courvoisier D. et al. THU0203 Real world effectiveness of baricitinib in the Swiss Rheumatoid Arthritis Register (SCQM-RA). In: Proceedings of the European Congress of Rheumatology, 3–6 June 2020, Virtual. Ann Rheum Dis 2020;79:325–6. [Google Scholar]
- 16. Burmester G, Fautrel B, Alten R. et al. A multinational, prospective, observational study in patients with rheumatoid arthritis receiving baricitinib, targeted synthetic or biologic disease-modifying therapies: 6-month effectiveness and patient reported outcome data from the European cohort. In: Proceedings from the ACR Convergence, 5–9 November 2021, Virtual. Arthritis Rheumatol 2021;73(suppl 9). https://acrabstracts.org/abstract/a-multinational-prospective-observational-study-in-patients-with-rheumatoid-arthritis-receiving-baricitinib-targeted-synthetic-or-biologic-disease-modifying-therapies-6-month-effectiveness-and-pat/ (13 December 2021, date last accessed). [Google Scholar]
- 17. Fitton J, Melville AR, Emery P, Nam JL, Buch MH.. Real-world single centre use of JAK inhibitors across the rheumatoid arthritis pathway. Rheumatology 2021;60:4048–54. [DOI] [PubMed] [Google Scholar]
- 18. Gilbert B, Courvoisier D, Mongin D. et al. POS0668 Real world effectiveness of baricitinib in the Swiss Rheumatoid Arthritis Register (SCQM-RA). In: Proceedings of the European Congress of Rheumatology 2–5 June 2021, Virtual. Ann Rheum Dis 2021;80:577–8. [Google Scholar]
- 19. Keystone EC, Taylor PC, Drescher E. et al. Safety and efficacy of baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate. Ann Rheum Dis 2015;74:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dougados M, van der Heijde D, Chen YC. et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis 2017;76:88–95. Erratum in: Ann Rheum Dis 2017;76:1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Genovese MC, Kremer J, Zamani O. et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016;374:1243–52. [DOI] [PubMed] [Google Scholar]
- 22. Nikiphorou E, Buch MH, Hyrich KL.. Biologics registers in RA: methodological aspects, current role and future applications. Nat Rev Rheumatol 2017;13:503–10. [DOI] [PubMed] [Google Scholar]
- 23. Fleischmann R, Schiff M, van der Heijde D. et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol 2017;69:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taylor PC, Keystone EC, van der Heijde D. et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2017;376:652–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided to Lilly by the British Society for Rheumatology under licence. Data will be shared upon reasonable request to the corresponding author with the additional permission of the British Society for Rheumatology.