Abstract
Messenger RNA (mRNA) is the template for protein biosynthesis and is emerging as an essential active molecule to combat various diseases, including viral infection and cancer. Especially, mRNA-based vaccines, as a new type of vaccine, have played a leading role in fighting against the current global pandemic of COVID-19. However, the inherent drawbacks, including large size, negative charge, and instability, hinder its use as a therapeutic agent. Lipid carriers are distinguishable and promising vehicles for mRNA delivery, owning the capacity to encapsulate and deliver negatively charged drugs to the targeted tissues and release cargoes at the desired time. Here, we first summarized the structure and properties of different lipid carriers, such as liposomes, liposome-like nanoparticles, solid lipid nanoparticles, lipid-polymer hybrid nanoparticles, nanoemulsions, exosomes and lipoprotein particles, and their applications in delivering mRNA. Then, the development of lipid-based formulations as vaccine delivery systems was discussed and highlighted. Recent advancements in the mRNA vaccine of COVID-19 were emphasized. Finally, we described our future vision and perspectives in this field.
KEY WORDS: mRNA, Lipid carriers, Drug delivery, Endosome escape, Nanoparticles, Encapsulation efficiency, Vaccine, COVID-19
Graphical abstract
Lipid carriers with high biocompatibility have excellent mRNA encapsulation and delivery efficacy. Meanwhile, the emergence of lipid carriers provides an effective solution for delivering mRNA vaccines.
1. Introduction
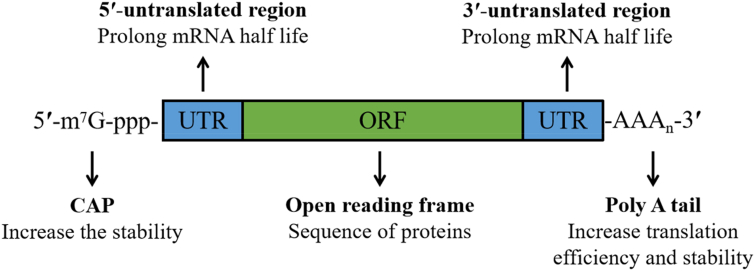
Messenger RNA (mRNA) is the template for protein biosynthesis. Natural mRNA is composed of a 5ʹuntranslated region (5ʹ UTR), open reading frame (ORF) and 3ʹuntranslated region (3ʹUTR) (as shown in Fig. 1)1. mRNA guides protein biosynthesis on ribosomes through the codon in the ORF. In vitro transcribed mRNA (IVT mRNA) shares a similar structure2. Delivering exogenous mRNA into cells can generate proteins instantaneously and accurately, offering an alternative to gene therapy3. Compared with other nucleic acid-based therapies, mRNA-based therapies do not need to enter the nucleus to function4. Once the mRNA reaches the cytoplasm, it translates immediately5. Furthermore, insertional mutagenesis is not risky because mRNA does not integrate into the genome6. IVT mRNA has become a promising candidate therapy for tumors, infectious diseases and protein replacement due to its safe and effective protein expression profile7. Regarding tumors and related infectious diseases, IVT mRNA can translate the corresponding antigens and activate or strengthen specific immunity, preventing infectious diseases8 or strengthening tumor immunity9. Current mRNA-based protein replacement therapies have focused on inherited metabolic diseases. Protein levels in patients can be restored using mRNA to express proteins lost due to genetic mutations10,11. Especially, rare genetic diseases, referring to a group of diseases caused by specific gene mutations, are one of the problems that modern medicine urgently needs to overcome12. Targeted delivery of mRNA has shown great potential in this field, including methylmalonic academia, ornithine transcarboxylasess deficiency and Fabry disease13,14. Now, most mRNA-based protein therapies are in preclinical studies. AZD8601, launched by AstraZeneca and Moderna, is the fastest trial and has completed patient enrollment in phase II, a low-dose cohort15,16. The therapeutic strategy of AZD8601 is to improve cardiac function and reduce heart damage by locally inducing VEGF-A protein expression to promote new angiogenesis in areas of the myocardia that undersupply blood10. In phase I clinical trials, AZD8601 showed good safety, and its intradermal injection temporarily increased skin blood flow while increasing local VEGF-A protein expression level, indicating the potential of promoting angiogenesis10.
Figure 1.
Schematic illustration of mRNA.
However, mRNA has inherent drawbacks, such as large size, negative charge, instability, hydrophilicity, etc17. The mRNA stability could be improved by structural modification18. Nevertheless, the membrane diffusion of mRNA is extremely poor due to its negative charges, high molecular weight and hydrophilicity. Consequently, intracellular delivery of mRNA is the critical factor limiting the application of mRNA-based therapies. Three challenges involve intracellular mRNA delivery: extracellular barrier, endosome escape and intracellular immunity19. In detail, mRNA is easily degraded by ribonuclease (RNase) in the body, so it is necessary to use an appropriate carrier to wrap mRNA to avoid degradation when delivering mRNA. Furthermore, the mononuclear phagocytic system (MPS) can systematically identify and eliminate foreign nanoparticles (NPs), resulting in NPs accumulating mainly in the liver and spleen and poor delivery to other tissues20. Then, when reaching the targeted cells, most mRNA carriers enter the cells by endocytosis21,22. Endocytic uptake localizes the cargo in early endosomes, which mature via late endosomes into endolysosomes23. Both cargo and carrier are degraded due to the acidic pH and lysosomal hydrolases in endolysosomes24. Therefore, mRNA requires release from endosomal vesicles before binding to ribosomes6. Finally, when foreign mRNA reaches the cytoplasm, it is recognized by Toll-like receptors 3 (TLR3) and TLR7/8 or other receptors to activate the natural immune system25. Although foreign mRNA can promote cellular or humoral immunity after mRNA immunization, this immunoreaction also compromises the effect of mRNA. For example, pattern recognition receptors (PRRs) in the TLR family can specifically recognize double-stranded or single-stranded mRNA and degrade it before it functions19. As a result, the efficient delivery of mRNA is fundamental to disease treatment.
In general, mRNA can be delivered using three main strategies: physical methods (disrupting the membrane barrier temporarily), viral vectors (utilizing biological modes of uptake) and non-viral vectors (reasonable design of nanocarriers)3,18,26. Physical methods are effective to some extent, especially electroporation27. Nevertheless, the physical methods are usually harmful to cells, therefore, unsuitable for use in vivo. Viral vectors are considered effective carriers to deliver mRNA; however, they are not extensively used due to limitations, such as small packing size, immunogenicity, cytotoxicity, carcinogenesis and production difficulties28, 29, 30, 31. Compared with viral vectors, non-viral vectors have lower immunogenicity and higher payload-capability and are easier to synthesize, making them the preferred vectors for mRNA delivery32, 33, 34. The most common non-viral carriers include lipids, polymers, and inorganic nanoparticles35, 36, 37. Here, we mainly discuss the lipid carrier-based mRNA delivery.
Lipid carriers can protect the mRNA from enzymatic degradation with a high encapsulation efficiency. Incredibly, some lipids are positively charged in the physiological environment and can be electrostatically coated with negatively charged mRNA, allowing the delivery system to associate well with the targeted cell membrane19. Furthermore, the NPs assembled from lipids can fuse with the endosomal membrane through a phospholipid bilayer, facilitating endosomal escape38. Here, this review summarizes the structure and properties of different lipid carriers and the mechanisms of mRNA delivery. At the same time, the status and clinical research of mRNA-based vaccines were introduced first, and then the development of mRNA vaccine in COVID-19 was emphasized. Eventually, we afford perspectives for lipid carrier-based mRNA delivery.
2. Lipid carriers for mRNA delivery
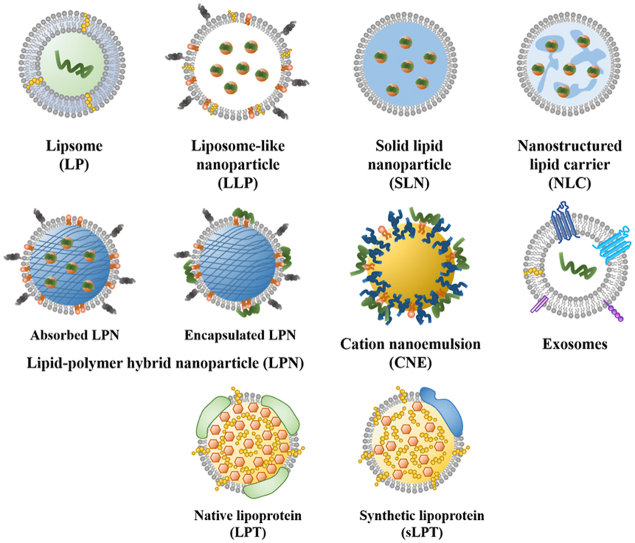
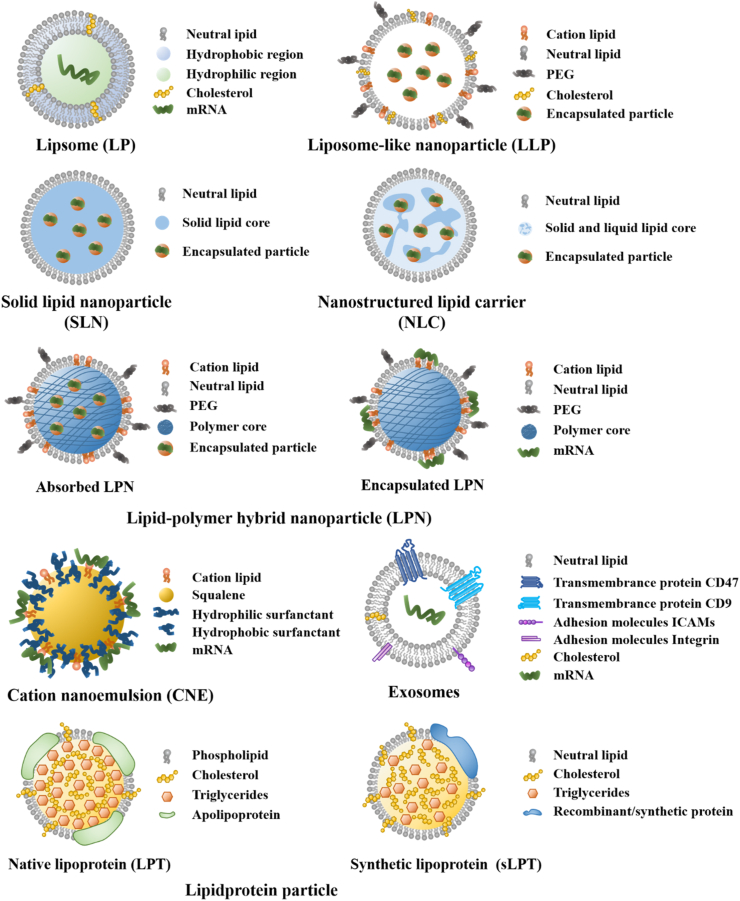
Lipid carriers possess a development history of more than 60 years39, aiming to improve drug delivery and reduce side effects40. Lipid carriers for mRNA delivery can be divided into several types, including liposomes (LPs), liposome-like nanoparticles (LLPs), solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), lipid-polymer hybrid nanoparticles (LPNs), nanoemulsions, exosomes and lipoprotein particles (LPTs)41. The characteristics of lipid carriers and their use for mRNA delivery are summarized in this section and Table 3 42,43. Meanwhile, the structures of different lipid carriers are shown in Fig. 2.
Table 3.
The comparison of lipid carriers for mRNA delivery.
| Carrier type | Characteristic | Advantage | Disadvantage |
|---|---|---|---|
| LPs | Spherical vesicles with a lipid bilayer. | Low toxicity, high biocompatibility, protection of envelope. | Low EE. |
| LPPs | Spherical vesicles with a cationic lipid bilayer. | Higher EE. | Significant toxicity. |
| LLPs | Sphere-shaped, multi/monolayer nanosized vesicle. | Less toxic, higher EE, improved endosome escape. | Instability under non-freezing conditions. |
| SLNs | Spherical vesicles with a core consisting of a solid matrix. | Higher physical stability, more robust mRNA protection, and higher delivery efficacy. | Expulsion of the incorporated mRNA. |
| NLCs | Spherical vesicles with a core consisting of both solid and liquid lipids. | Higher physicochemical stability, higher drug-loading capacity, less drug leakage, and controlled drug release. | In the preparation (high-pressure homogenization) process, the high temperature may promote the degradation of drug and the carriers. |
| LPNs | Core-shell nanoparticle structure composed of polymer core and lipid shell. | Less biofouling, prolonged half-life, sustained drug release. | Polymeric materials may bring unwanted side effects. |
| Nanoemulsions | A heterogeneous colloidal dispersion of nanodroplets dispersed in another liquid phase. | Prolonged half-life, less irritation. | Emulsifier incorporation may generate safety risks. |
| Exosomes | Nanosized lipid vesicles secreted from living cells, ranging from 30 to 150 nm. | Extremely high biocompatibility and stability, biological barrier permeability, and extended circulation time. | Modification is required to obtain high target site affinity. The preparation of exosomes is time-consuming with high costs. |
| LPTs | Heterogeneous NPs present in biological fluids, mainly composed of lipids and proteins. | Extremely high biocompatibility, non-immunogenicity and stability, intrinsic targeting ability. | The purification of LPTs is time-consuming and poorly scalable. |
These lipid carriers have lower toxicity and higher inclusion protection than non-lipid vectors. The advantages and disadvantages of the carriers were demonstrated by comparing with LPs.
EE, encapsulation efficiency; LPs, liposomes; LPNs, lipid-polymer hybrid nanoparticles; LLPs, liposome-like nanoparticles; LPPs, lipoplexes; LPTs, lipidprotein-particles; NLCs, nanostructured lipid carriers; SLNs, solid lipid nanoparticles.
Figure 2.
Schematic illustration of different lipid carriers.
2.1. LPs
LPs, a closed phospholipid bilayer spherical vesicle system composed of amphiphilic molecules, are able to carry hydrophobic/hydrophilic molecules41,44,45. Since this closed bilayer phospholipid structure was first observed in 196146, LPs, as an attractive lipid carrier, are widely used for drug delivery to combat various diseases, such as tumor, fungal and bacterial infection47. So far, over 20 liposome products have been approved for the clinic, for example, Doxil®, Vyxeos®, Lipusu®, Ambisome®, Arikayce®, DepoDur™, etc48. Doxil®, doxorubicin liposomes, is the first U.S. Food and Drug Administration (FDA)-approved nanomedicine to treat the recurrent ovarian tumor and the human immunodeficiency virus (HIV) related Kaposi's Sarcoma49, 50, 51. The liposomes can prolong blood circulation time, effectively load doxorubicin and passively target tumors. Accurately, modern mRNA delivery systems derive from these traditional liposomal formulations for small-molecule therapeutics. Now LPs are a effective delivery vector for mRNA due to their low toxicity and high biocompatibility advantage, as LPs are analogs of biological membranes, which can be composed of natural and synthetic phospholipid preparation52. In addition, mRNA encapsulated in LPs is not easily degraded by RNase. Commonly used lipid materials for mRNA delivery are shown in Supporting Information Table S1 3,53,54. Martinon et al.55 encapsulated the mRNA encoding the influenza virus nucleoprotein into negative LPs comprised of cholesterol (chol), dipalmitoylphosphatidylcholine (DPPC), and phosphatidylserine (PS). Despite the low encapsulation efficiency, the LPs induced the proliferation of anti-influenza cytotoxic T lymphocytes in vivo.
In order to improve the interaction with LPs, mRNA can be formulated with cationic LPs to form lipoplexes (LPPs). The complexation of negatively charged mRNA with positively charged cationic LPs increases the encapsulation efficiency of mRNA. Moreover, cationic LPs could bind to the cell membrane, assisting mRNA entering the cell56. Cationic lipids, the main components of cationic LPs, share a similar structure with natural lipids, except for the presence of a cationic head group57. Historically, N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA) was the first synthetic cationic lipid used to deliver mRNA58. DOTMA combined with 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) was commercialized as Lipofectin59. 1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP), as a DOTMA derivative, is a cationic lipid-containing monounsaturated fatty acid with low preparation cost and high mRNA delivery efficiency60. Therefore, it has become a widely studied cationic lipid material. DOTMA and DOTAP have been used for mRNA delivery alone or in combination with other materials. For example, spleen-targeted DOTMA-mRNA LPPs has been designed as mRNA vaccine to treat autoimmune encephalomyelitis61. This vaccine induced the proliferation of antigen-specific CD4+ T cells. The results indicated that immunosuppression was enhanced, and mice's pathological symptoms were relieved. This method provided ideas for the clinical translation of antigen-based therapy for autoimmune diseases. For another, most researchers modify LPs to improve the delivery capacity of the carrier. In order to increase the carrier concentration on the cell surface and thus enhance the uptake and transfection potency of mRNA, Zohra et al.62 used carbonate apatite, an inorganic crystal with pH sensitivity and strong affinity for mRNA, to form a complex between the LPs formed by DOTAP. The results showed that the mRNA-DOTAP-carbonate apatite complex could significantly elevate mRNA expression in mitotic cells compared with the control group. Wherein carbonate apatite could reinforce the transport of cationic LPs and mRNA due to a simple physical phenomenon: the mRNA-DOTAP-carbonate apatite complex precipitate on the cell surface by gravity. Zhang et al.63 prepared DOTAP LPs decorated with chol-modified cationic hydrophilic peptide DP7-C, aiming to increase the delivery efficiency of mRNA encoding individualized neoantigens to dendritic cells (DCs) and enhance the activation of DCs. The ternary complex consisting of cationic LPs-cationic peptide-mRNA was called lipopolyplexes (LPR)1. DP7-C in the complex has low cytotoxicity and potential immunomodulatory effects and could effectively load antigen into cells through the caveolin and clathrin-dependent pathway. In addition, DP7-C can stimulate DCs maturation using the TLR2-MyD88-IKK-IκB–NF–κB signaling pathway and boost the immune response to the neoantigen-loaded DCs vaccine64. The results displayed that the LPR promoted DC maturation and cytokine secretion, enhancing antigen-specific lymphocyte response and anti-tumor effect. The complex had dual carrier and immune adjuvant functions, potentially improving the intracellular mRNA delivery and immune response. Protamine is a basic protein in the nucleus of mature sperm of fish, which can condense mRNA into nano-complex to raise mRNA's stability and encapsulation efficiency. Zhang et al.65 used protamine-DOTAP cationic LPs to deliver mRNA encoding the Survivin-T34A gene, demonstrating a significant therapeutic effect on various C26 colon cancer cell models in vitro and vivo. Protamine in the complex was employed to concentrate the mRNA into nanosized particle and protect it from nuclease degradation. Whereas cationic lipids have significant toxicity, affecting clinical application66,67. New materials are urgently needed to solve this issue.
2.2. LLPs
LLPs, always termed lipid nanoparticles, are sphere-shaped and nanosized vesicles commonly composed of ionizable lipids, phospholipids, chol and polyethylene glycol (PEG)–lipid conjugate68. In 2018, the first small interfering RNA (siRNA) drug, patisiran (trade name: Onpattro®), encapsulated by LLPs, was approved by the FDA to treat hereditary transthyretin-mediated amyloidosis2,69. It is considered a milestone for the LLPs delivery system. After that, LLPs have been increasingly applied for mRNA delivery.
Generally, each lipid in LLPs performs a unique function. Lipids containing tertiary amine groups, such as Dlin-MC3-DMA, C12-200, etc., are ionizable lipids that favor mRNA encapsulation and endosomal escape57,70. The early-use cationic lipids are positively charged under physiological conditions, readily interacting with other negatively-charged molecules and easily captured by immune cells in the physiological environment71. By contrast, ionizable lipids with pH sensitivity are less toxic than nonionizable cationic lipids because as the pH changes in vivo, these lipids are only positively charged in the cell and not charged in the bloodstream72. Meanwhile, when the pH value is lower than the apparent pKa value of the ionizable lipids, ionizable lipids become positively charged and enable efficient encapsulation of mRNA in LLPs through electrostatic interactions with the negatively charged mRNA. During the endosomal escape, as endosomes mature, pH drops below the apparent pKa of ionizable lipids; consequently, most ionizable lipids are protonated73. Ionizable lipids interact with anionic lipids on the endosomal membrane to form destructive non-bilayer structures that eventually release the encapsulated mRNA into the cytoplasm19,74. In mRNA vaccines, ionizable lipids may act as adjuvant agents. Recently, a study investigated the adjuvant effect of ionizable lipids by administering mRNA-LLPs to mice: the ionizable lipids produced the auxiliary effect by activating interleukin-675. Another study screened 30 LLPs that differed only in ionizable lipids structure to encapsulate mRNA encoding H10N8 influenza hemagglutinin antigen. The immunogenicity produced in mice varies, indicating that mRNA-LLPs' immunogenicity depends highly on ionizable lipid structure76. Interestingly, an optimal lipid pKa for intramuscular injection (im) immunogenicity is between 6.6 and 6.977. This differs from the optimal lipid pKa range (6.2–6.6) for intravenous injection (iv) immunogenicity77. Indeed, the relationship between ionizable lipids and LLPs-induced immunogenicity is still ambiguous.
Over the past few decades, the design of ionizable lipids has undergone significant improvements. When the first ionizable lipid, 1,2-dioleoyloxy-3-(dimethylamino)propane (DODAP), was applied for nucleic acid delivery, the transfection efficacy was modest78. Subsequently, new ionizable lipids were developed, including DLinDMA, DLin-KC2-DMA, DLin-MC3-DMA (used in Onpattro®), SM102, and ALC-031579,80. Two ionizable lipids, SM-102 and ALC-0315, have been used in two COVID-19 vaccines: mRNA-1273 and BNT162b2. Traditional PEGylated lipids, the lipid component with the smallest mole percentage in LLPs, affect the size and dispersion of LLPs and improve the bilayer stability and nonspecific cellular uptake60,71,81. Traditionally, ionizable lipids are mainly screened by synthesizing numerous lipids and testing their effectiveness in vivo, which requires high cost, time and materials82. Wang et al.83 attempted to apply computational methods to accelerate the development of ionizable lipids and then developed the first machine learning prediction model for LLP-based mRNA vaccines. This model can be applied for virtual screening of LLP preparations in the future. PEG chains form a hydrophilic steric barrier on the surface of LLPs, promoting LLPs’ self-assembly and preventing aggregation to ultimately improve stability84,85. Lokugamage et al.68 designed LLPs for lung delivery of mRNA via nebulization. The results demonstrated that lacking PEG-lipids produced unstable LLPs with elevated polydisperse index. Whereas LLPs containing PEG-lipid improved mRNA delivery to the lung. Furthermore, PEG-lipids affect the mRNA encapsulation efficiency, circulation time and immune response86,87. PEGylation is a widely used method to prevent rapid clearance and increase the circulation time of NPs86. For instance, within the anticancer drug Doxil®, distearoyl phosphoethanolamine-PEG (DSPE-PEG) is included to limit protein binding and clearance by the MPS, thus prolonging circulation time, thereby increasing tumor accumulation of doxorubicin and reducing cardiotoxicity88. However, an accelerated blood clearance (ABC) phenomenon has been observed in PEGylated NPs, demonstrating rapid clearance in vivo after repeated injections41,89,90. Besin et al.91 found that LLPs also had the ABC phenomenon by producing anti-phosphatidylcholine and anti-PEG antibodies. Fortunately, the ABC phenomenon has not seriously disturbed the clinical use of PEG anticancer drugs92. In addition to DSPE-PEG, dimyristoyl phosphatidylethanolamine-PEG (DMPE-PEG) and 1,2-dimyristoyl-rac-glycero-3-methoxy polyethylene glycol (DMG-PEG) are also commonly utilized. As the skeleton of LLPs, phospholipids [distearoyl phosphatidylcholine (DSPC) and DPPC] contribute to the formation of the lipid bilayer and the transition from lamellar to hexagonal phase during the endosome, promoting the cytosol release of mRNA60. However, due to its well-known stability, only DSPC is included in the commercial LLPs. Chol plays a role in stabilizing the lipid bilayer and reducing the phase transition temperature, facilitating the stratified transition to hexagonal phase60. In LLPs formulations, chol can significantly decrease the amount of surface binding proteins, prolonging the circulation half-life93. In addition, chol facilitates mRNA encapsulation. Increasing chol leads to increased membrane rigidity, reducing drug leakage from the liposomal core.
The structure of LLPs-mRNA is unlike that of LPs-mRNA. For LLPs-mRNA, mRNA is complexed with ionizable cationic lipids in acidic circumstances and loaded in the aqueous cores of LLPs, while mRNA is encapsulated directly in the hydrophilic core of LPs94. Small-angle scattering determination demonstrated that LLPs have a disordered inverse hexagonal internal structure in the presence of mRNA70. Furthermore, test by contrast variation small-angle neutron scattering indicated that DSPC, PEG-lipids, as well as part of ionizable lipids and chol, are localized mainly on the surface of LLPs. Major parts of ionizable lipids, chol, water and mRNA reside inside the core. The results implied that the mRNA is partially exposed to the water inside the LLPs, causing instability under non-freezing conditions70. Interestingly, Sebastiani et al.95 found that the outer shell of mRNA-LLPs is a monolayer, while other researchers proposed that the outer shell consists of one or multiple bilayers96. These findings suggest that mRNA-LLPs possess several structures, likely depending on lipid properties and the preparation procedure.
For the intracellular delivery, the entrapment of acidic endosomes allows LLPs to have a positive charge, facilitates the association with the endosome membrane and disrupts the bilayer structure, enabling an increased release of mRNA into the cytoplasm compared to LPs97. Meanwhile, during the endosomal escape, the ionizable lipids are prone to form an inverted hexagonal phase structure and destroy the endosomal membrane53. Therefore, LLPs are more effective for intracellular delivery than LPs. Overall, LLPs have become a relatively mature mRNA carrier because of the strengths of high encapsulation rate, low toxicity, enhanced cell uptake, high stability and prolonged circulation time. Even so, LLPs’ stability and storage conditions are still challenging.
2.3. SLNs and NLCs
Unlike LPs, SLNs and NLCs have been developed with more complex internal architectures and higher physical stability57,98. SLNs are tiny spherical vesicles with a solid lipid core surrounded by a layer of surfactants as stabilizers42. Whereas NLCs have a solid and liquid lipid core42. The SLN core is coated by triglycerides, steroids and fatty acids, remaining solid at room temperature99,100. SLNs have more robust mRNA protection and better ability to prevent leakage because their solid core allows only a little water to be loaded101. Meanwhile, SLNs prepared with several techniques (i.e., high-pressure homogenization) implemented in the pharmaceutical industry show long-term stability and the possibility of being subjected to commercial sterilization and lyophilization procedure102. Gomez-Aguado et al.103 employed a combination of cationic and ionizable lipids to construct SLNs by the solvent emulsification-evaporation for delivering mRNA, aiming to evaluate the influence of formulation on the mRNA delivery efficiency by studying the transfection efficiency, protein expression and intracellular disposition of mRNA in human retinal pigment epithelial cells (ARPE-19) and human embryonic kidney cells (HEK-293). The formulation that involved SLNs, mRNA, the cationic peptide protamine and a polysaccharide showed the highest delivery efficacy. And SLNs with DOTAP exhibited good long-term stability.
Over time, the maturation of the crystalline structure of SLNs gives rise to the expulsion of the incorporated mRNA104,105. Moreover, many studies have shown that numerous drugs may attach or strand on the outside layer surface rather than the solid core, raising questions about the drug delivery efficacy and stability of SLNs43,106. NLCs are considered an advanced version of SLNs107. The addition of liquid lipids to NLCs tolerates structural rearrangement of the matrix108. Liquid lipids reduce the crystalline degree of lipid core, thereby avoiding drug leakage and increasing drug loading and physicochemical stability109. Due to the presence of solid and liquid lipids, NLCs can encapsulate components in both solid and liquid phases and control drug release rates110. Gerhardt et al.111 demonstrated the thermal stability and adaptability of the NLCs-mRNA delivery system. They found only liquid NLCs were stable at refrigerated temperature for more than 1 year. NLCs complexed with mRNA could be lyophilized and stored at room temperature for more than 8 months. This finding potentially addresses the restriction that LLPs need to be stored at −20 °C or −70 °C, improving formulations’ transport and storage capacity.
2.4. LPNs
Polymers (polymer NPs, micelles and dendrimers) and lipid carriers (LPs, LLPs, etc.) can effectively deliver drugs. Polymer NPs are prepared from natural polymers such as chitosan or synthetic biodegradable and biocompatible polymers such as polylactic-glycolic acid (PLGA)112,113. The core–shell structure of polymeric NPs enables them to encapsulate water-insoluble drugs, reducing the biofouling of NP, extending the circulating half-life and controlling the release of the cargo. However, polymer NPs still have some disadvantages, including the risk of particle aggregation and toxicity114,115. Compared with polymer NPs, LPs have been considered more promising drug delivery vehicles because of their excellent biocompatibility52. However, the conventional LPs are quickly cleared by the reticuloendothelial system (RES), resulting in their poor bioavailability; and the LPs are prone to leakage of their contents during drug delivery116. In order to solve the limitations of polymer NPs and LPs, a new generation of therapeutic agent delivery carriers called LPNs has been developed. LPNs are a core–shell nanoparticle structure composed of polymer core and lipid shell and integrate the physicochemical properties and biocompatibility of lipids and polymers117. The polymer core can effectively load the therapeutic drug; the lipid layer wraps the polymer core, confers biocompatibility, and act as a molecular fence to minimize leakage of the encapsulated contents during LPNs preparation118. In addition, the lipid layer slows the polymer degradation rate of the LPNs by limiting inward water diffusion, enabling sustained drug release kinetics. The outer layer lipids can be coupled with PEG to extend the blood circulation time of LPNs117.
The research of LPNs mainly focused on polymer designing to enhance RNA delivery. Zhao et al.119 synthesized anN1,N3,N5-tris (2-aminoethyl) benzo-1,3,5-tricarboxamide (TT)-derived lipid-like nanomaterial (TT3-LLNs) for delivering multiple types of mRNA. The LPNs obtained by co-preparation of PLGA4 (MW 24,000–38,000 g/mol) with TT3-LLNs significantly increased the mRNA delivery efficiency, approximately 2-fold over that of using TT3-LLNs alone.
Compared with PLGA polymers, incorporating cationic or ionizable lipids can significantly enhance the loading efficiency of mRNA. Meyer et al.120 prepared ionizable LPNs using PLGA, cationic lipid DOTAP and ionizable lipids D-Lin-MC3-DMA through a microfluidic mixing strategy. These LPNs could bind mRNA efficiently. The positive surface charge of LPNs increased as the percentage of cationic lipids increased, while the charge decreased with increasing mRNA dose. The loading efficiency of mRNA in LPNs prepared with DOTAP is high, ranging from 80% to 100%120. LPNs prepared with DOTAP/MC3 mixture showed 80%–100% mRNA loading efficiency at high DOTAP concentrations. In contrast, at high MC3 concentrations, the loading efficiency decreased to 30%–50%, likely owing to the transition of MC3 from positively charged to neutral after increasing pH, reducing the ability to bind mRNA. Yasar et al.121 prepared LPNs coated with cationic lipid DOTMA by optimizing NPs composed of PLGA. The LPNs significantly improved transfection compared to polymer/mRNA particles, an elevation that approached 80%, while the transfection effectiveness of chitosan-PLGA NPs was only 5%121. LPNs can not only encapsulate mRNA in the polymer core but also are able to load mRNA on the surface of LPNs through an adsorption strategy. For instance, Su et al.122 prepared LPNs with a pH-sensitive poly (β-amino ester) (PBAE) core coated by the phospholipids of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), DOTAP and DSPE-PEG and then loaded mRNA onto the surface of the LPNs by electrostatic adsorption. In vitro experiments demonstrated that the LPNs/mRNA complex was readily taken up by DCs and could deliver mRNA into the cytoplasm, with 30% translation efficacy. The two approaches to load mRNA in LPNs have their advantages. The adsorption strategy showed higher mRNA expression levels in tissues in vivo, whereas the encapsulation approach could protect the mRNA and have a controlled release effect120. In practical applications, the strategy of mRNA loading in LPNs should be amended according to therapeutic purposes.
Various combinations of lipids and polymers have been extensively studied to construct LPNs for siRNA delivery, but little is known about the effect of different polymers on lipid-mediated mRNA delivery. Whether incorporating polymeric materials as cores brings unwanted side effects compared to pure lipid materials has not been reported in detail. Therefore, although LPNs have shown the potential to deliver mRNA, further research is urgently required.
2.5. Nanoemulsions
Nanoemulsions comprise a heterogeneous colloidal dispersion of nanodroplets dispersed in another liquid phase123,124. Nanoemulsions are mainly applied to load active molecules in the lipid core, protecting drugs from degradation and prolonging the drug's half-life in plasma125,126. An emulsifier is added to the formulation for the droplet dispersion in the aqueous phase. Emulsifiers are compounds with an amphiphilic character being able to reduce the interfacial tension between two immiscible phases125. Lipid nanoemulsions are distinguished from traditional lipid particles. Nanoemulsions are divided into oil-in-water (O/W) nanoemulsion and water-in-oil (W/O) nanoemulsion127. O/W lipid nanoemulsions consist of an inner oil core and a monolayer of phospholipids and emulsifiers128. Insoluble drugs are dissolved in the inner liquid oil phase, and phospholipids wrap the outer layer; therefore, the nanoemulsion can encapsulate lipophilic drugs in an aqueous solution and improve the drug stability129. W/O nanoemulsion consists of an internal phase of water droplets and an external oil phase130. Similar to LPs, the components in nanoemulsions are highly biocompatible and induce less toxicity or irritation in vivo42. Therefore, nanoemulsions are effective drug delivery vehicles.
The most utilized nanoemulsion for gene delivery is cationic nanoemulsions (CNEs) and are prepared by different methods, such as high-pressure homogenization, microfluidization, and sonication131. The technology used in the manufacturing process, lipid type selection, and formulation parameters significantly affect CNE properties, including droplet size, surface charge, and decomplexation kinetics of nucleic acids131,132.
For gene delivery, CNEs typically contain cationic components complexed with nucleic acids and a hydrophobic oily core surrounded by phospholipids and other surfactants. Significant progress has been made in the preclinical and clinical development of CNEs to deliver pDNA133,134, miRNA135, siRNA136 and mRNA137. For immune response enhancement, emulsion adjuvants could be formulated in CNEs, such as MF59 and AS03. Recently, several researchers summarized the emulsion adjuvants approved and under development (Table 1)138,139. For mRNA delivery, the drug is bound to the CNE surface140. Brito et al.137 developed a CNE formulation to deliver a 9 kb self-amplifying mRNA vaccine (saRNA). The CNEs contain the cationic lipid DOTAP and are emulsified with a safe emulsion adjuvant, MF59. The prepared CNE could protect the mRNA from degradation by RNase in vivo137. The results demonstrated that the CNE vaccine had comparable protein expression efficacy to a typical viral vector and had well tolerated and immunogenic properties in several animal types, including rabbits, rats, mice and nonhuman primates137. Compared with traditional mRNA vaccines, saRNA vaccines can self-amplify in the body and elevate antigen expression. Therefore, robust immune responses were displayed at low saRNA doses (saRNA dose was typically 100 times lower than traditional mRNA vaccines)141. HIV saRNA vaccine technology delivery using CNEs was proven safe and immunogenic in a rhesus monkey animal model, eliciting both humoral and cellular immune responses142. In addition, a novel rabies self-amplifying mRNA vaccine was established using CNEs, stimulating potent neutralizing antibody responses in rats. Using the vaccine delivered by CNEs, the injected saRNA is expected to enter the host cell and express the target antigen locally. A cationic nanoemulsion (NCT04062669) is currently in clinical trials for rabies virus143.
Table 1.
Emulsion adjuvants approved and under development.
| Name | Time of approval | Clinical development stage | Formulation | Mechanism or receptor | Immune profile | Adverse reaction |
|---|---|---|---|---|---|---|
| MF59 | 1997 | Licensed for seasonal and pandemic flu vaccine | Squalene oil, span 85, Polysorbate 80 | Immune cell recruitment and antigen uptake | Antibody, Th1, Th2 | Local reactions and inflammatory arthritis |
| AS03 | 2009 | Licensed for pandemic flu vaccine, phase III for COVID-19 | Squalene oil, α-tocopherol, polysorbate 80 | Immune cell recruitment, antigen uptake | Antibody, Th1, Th2 | Injection-site pain, fatigue, headache, and muscle aches |
| AF03 | 2010 | Licensed for pandemic flu | Squalene oil, span80, eumulgin B1, mannitol | Immune cell recruitment and antigen uptake | Antibody, Th1, Th2 | Fever and erythema |
| CoVaccine HT | 2022 | Phase III for COVID-19 | Squalane oil, polysorbate 80, sucrose fatty acid sulfate esters | Little is known | Antibody, Th1 | Produce pro-inflammatory cytokine, exacerbates immunopathology |
| GLA-SE/SLA-SE | – | Phase I – phase III for tuberculosis, schistosomiasis, leishmaniasis | Squalene oil, poloxamer188 and synthetic phosphatidylcholines | TLR4 | Antibody, Th1 | Increased levels of pro-inflammatory cytokines |
| Sepivac SWE | – | Preclinical, phase I for COVID-19 | Squalene oil, span85 and polysorbate 80 | Immune cell recruitment and antigen uptake | Antibody, Th1, Th2 | Local reactions |
GLA-SE, glucopyranosyl lipid adjuvant-stable emulsion; SE, stable emulsion; SLA-SE, synthetic lipid A stable emulsion; SWE, squalene-in-water emulsion.
‒, not applicable.
2.6. Exosomes
Extracellular vesicles (EVs) secreted by all cell types in the organism can transmit biologically active molecules, including proteins and nucleic acids, and regulate the communication between cells144, 145, 146. According to biogenesis, EVs can be generally divided into three populations: exosomes, microvesicles and apoptotic bodies147,148. Exosomes are 30–150 nm vesicles originating from the fusion of multivesicular endosomes with the plasma membrane3, and the 30–100-nm exosomes were used in most studies144. Exosome membranes are enriched in lipids and proteins, qualifying them as a drug delivery system for mRNA149. Firstly, exosomes can intrinsically carry and protect nucleic acids150,151. Valadi et al.152 confirmed 1,300 different mRNAs in the exosomes isolated from a mast-cell line. Secondly, specific proteins on the surface of exosomes, such as transmembrane protein CD47, are “don't eat me” signals, avoiding the MPS and decreasing the clearance from the circulation153,154. A study indicated that intravenously-injected exosomes had similar clearance with LPs composed of phosphatidylcholine and chol155. Thirdly, lipid components and protein components enable exosomes to cross cellular barriers and then release mRNA to the cytosol by endogenous mechanisms. Several mechanisms have been hypothesized to describe the interactions of exosomes and recipient cells152, i.e., ligand–receptor interactions156, fusion with target-cell membrane157 and endocytosis158. However, the mechanism by which exosomes release the cargo into the cytoplasm and the fate of exosomal components in recipient cells are still unknown.
mRNA can be loaded into exosomes by direct loading and indirect loading3,159. For direct loading, pDNA coding the desired mRNA is used to transfect cells and then mRNA-loaded exosomes are isolated. For indirect loading, exosomes are co-cultivated with cells transfected by pDNA, and then bulk electroporation (BEP) is employed to create transient pores in the membrane of exosomes to facilitate the migration of mRNA from cells to exosomes160, 161, 162. Exosomes prepared in these two manners could be stored at −80 °C. Compared with indirect loading, direct loading seems to be easier to operate with a lower cost.
Nonetheless, unmodified exosomes delivered systemically prefer to accumulate in the liver, kidney, and spleen, causing a low concentration of mRNA in targeted tissues163,164. For the improvement of mRNA delivery, exosomes could be bioengineered by inserting tissue-special coding sequences or combining natural exosomes with peptides. Tissue-specific miRNAs could regulate mRNA at the translational level, allowing the mRNA only to work in the targeted tissues165. Sun et al.166 encapsulated tissue-specific miRNA-controlled mRNA into exosomes to increase targeted delivery efficiency. To obtain engineered mRNA, they transfected HEK293T cells with a plasmid coding PGC1α possessing two miR-148 binding sites, in which PGC1α is an essential transcription factor for fat browning167 and miR-148 is adipose tissue-specific miRNA168. In vitro study showed that engineered mRNA was successfully encapsulated into exosomes and was translationally activated by corresponding miRNAs in the recipient cells. In vivo experiment, significantly increased PGC1α protein expression was found in the adipose tissue of mice treated with exosomes loaded with miR-148-PGC1α, which was much lower in the lungs and other tissues166. Besides tissue-specific expression of the delivered genes, the modified exosomes hold promise for increasing delivery to targeted cells. Yang et al.169 cloned glioma-targeting peptides (a CDX peptide for U87 targeting and a CREKA peptide for GL261 targeting) into the N-terminal of CD47 to produce targeted exosomes (Exo-T). The uptake of Exo-T in U87 and GL261 cells was dramatically increased as well as the translation of mRNA. Compared to non-targeted exosomes, Exo-T could cross the blood–brain barriers and deliver mRNA efficiently to U87 cells, inhibiting tumor growth and prolonging survival. Moreover, immunohistochemical staining results confirmed that Exo-T treatment had little effect on other tissues examined.
In summary, exosomes exhibit appealing properties for mRNA delivery, including biocompatibility, stability in the circulation and biological barrier permeability170,171. Bioengineered exosomes show more significant potential in disease treatment than natural exosomes due to improved performance in vivo. So far, few clinical applications of nanocarriers based on exosomes have been reported. One possible reason is that preparing engineered or modified exosomes is time-consuming with high costs. With a deeper understanding of exosomes, several physical approaches, such as ultrasound, could assist the exosome delivery of mRNA166. Finally, given that natural exosomes derived from different cells have different lipids and proteins, the selected components of natural exosomes, such as CD47, could be formulated into other lipid carriers to enhance stability, targeting, and uptake144.
2.7. LPTs
LPTs, heterogeneous NPs present in biological fluids, mainly composed of lipids (triglycerides and chol) and proteins (apolipoprotein), play an essential role in the metabolism of lipids172. Due to different ratios of lipids and proteins, LPTs have an extensive range of size and density173. LPTs can be divided into five subclasses: chylomicrons, very low-density lipoproteins (VLDLs), low-density lipoproteins (LDLs), intermediate-density lipoproteins (IDLs), and high-density lipoproteins (HDLs) (Table 2), according to the origin, size and density174. Similar to EVs, the native origin of LPTs ensure many benefits for drug delivery, such as biocompatibility, non-immunogenicity and stability in circulation175. LPTs can transport lipids from intestines to other tissues for storage and move lipids from other tissues to the liver for metabolism, implying their intrinsic targeting ability in vivo174. For example, during the transport of lipids, LDLs and HDLs can bind to receptors on specific cells, such as the LDL receptor (LDL-R), scavenger receptor class B type 1 (SRB1) and adenosine triphosphate-binding cassette transporter subfamily G member 1 (ABCG1)90,175. Significantly, some of these receptors are overexpressed on tumor cells and atherosclerotic plaques, allowing LDLs and HDLs a promising vehicle for small molecule drugs to treat cancers and cardiovascular diseases176, 177, 178.
Table 2.
Classification of lipoproteins.
| Lipoprotein subclass | Origin | Density (g/cm3) | Size (nm) | Ref. |
|---|---|---|---|---|
| Chylomicrons | Intestines | <0.950 | 80–1000 | 195 |
| VLDLs | Liver, intestines | 0.930–1.006 | 30–80 | 196 |
| IDLs | Catabolism of VLDLs | 1.006–1.019 | ∼25 | 196 |
| LDLs | Catabolism of IDLs | 1.019–1.063 | ∼20 | 197 |
| HDLs | Liver, intestines | 1.063–1.210 | 5–10 | 174 |
IDLs, intermediate-density lipoproteins; LDLs, low-density lipoproteins; HDLs, synthetic HDLs; VLDLs, very low-density lipoproteins.
Yet, there are various other particles in biological fluids, such as EVs, possessing a similar size and density to LPTs, leading to difficulty separating LPTs from other components179,180. Besides, the purification of LPTs is time-consuming and poorly scalable, hindering their use in drug delivery181. Synthetic lipoproteins (sLPTs) are composed of proteins (recombinant or synthetic origin) and synthetic lipids or other hydrophobic compounds175. The strategies for sLPT synthesis include vortex182 and microfluidic technology183, followed by purification using dialysis or ultracentrifugation184,185. sLPTs demonstrate similar properties, such as size, density and shape, to natural LPTs, maintaining the advantages of biocompatibility, stability and targeting ability184. So far, synthetic HDLs (sHDLs) and synthetic LDLs (sLDLs) have been applied to deliver RNAs. After modification with hydrophobic components, such as chol or cationic lipids, RNAs could co-incubate with sLPTs for targeted delivery186,187. Nakayama et al.186 prepared sHDLs and sLDLs to deliver chol-conjugated siRNAs (chol-siRNA) for atherosclerosis therapy. sHDLs were formulated from recombinant apolipoprotein A1 (apoA) and palmitoyloleoylphosphatidylcholine (POPC), called A-sLPTs, while sLDLs were composed of apolipoprotein E3 (apoE) and dimyristoylphosphatidylcholine (DMPC), named E-sLPTs186. In vivo studies showed that silencing apolipoprotein B (apoB) mRNA was enhanced by administering chol-siRNA/sLPTs complex. E-sLPTs could deliver chol-siRNA to mouse liver with higher efficacy than A-sLPTs because E-sLPTs were not significantly affected by high endogenous levels of LDLs in plasma, indicating the potential as carriers for lipophilic conjugates such as chol-siRNA186.
At present, sHDLs and sLDLs with a diameter of less than 20 nm have been applied to deliver microRNA188,189 and siRNA190,191 rather than mRNA, probably owing to the difference in the payload size (siRNA is 20–30 nucleotides vs. mRNA is at least 1,000 or more nucleotides) or the flexibility (siRNA is a rigid duplex vs. mRNA is a flexible single strand)192, resulting in poorly loading mRNA for sLPTs. Moreover, mRNA might form local secondary structures depending on the nucleotide sequence and compromise the complexation with sLPTs192, 193, 194.
3. Lipid carriers for mRNA vaccine delivery
3.1. Application of lipid carriers for mRNA vaccine delivery
mRNA was initially proposed in 1989 as a drug to treat diseases, demonstrating that mRNA could be directly delivered and expressed in eukaryotic cells under the packaging of the cationic lipid DOTMA198. Subsequently, mRNA was injected into mouse skeletal muscle, and effective expression was observed in vivo, offering the preliminary foundation for mRNA as a vaccine platform and proving the feasibility of mRNA vaccine development199. mRNA vaccines are divided into non-replicating mRNA vaccines and self-amplifying mRNA vaccines200. Non-replicating mRNA vaccines are composed of conventional or base-modified non-amplifying mRNA molecules. Self-amplifying mRNA vaccines are constituted of saRNA, also known as replicon able to self-amplify201. Compared with non-replicating mRNA vaccines, this sequence-optimized "next-generation” vaccine allows cells to synthesize proteins similar to the viral replication process201. Each mRNA sequence can be replicated multiple times in the cell before it is translated into protein, increasing the protein expression and strengthening the vaccination202.
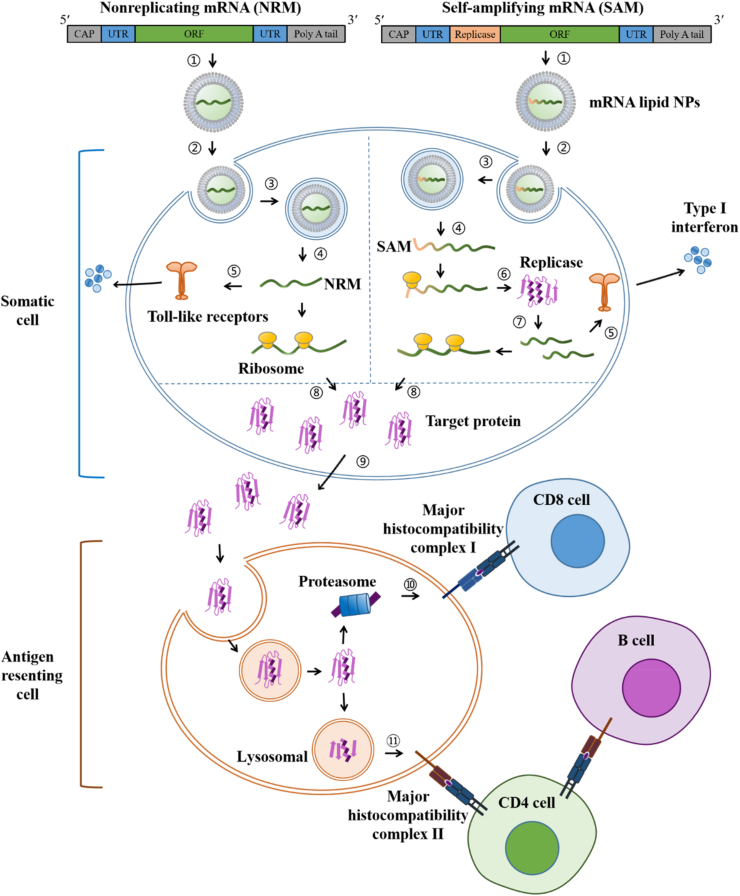
The carrier-based mRNA vaccines work by delivering the mRNA-expressing antigen target into the body, translating and expressing the protein in vivo, and stimulating the body to produce a specific immune response and immune protection (Fig. 3)203. E.g., mRNA lipid NPs are usually internalized by somatic cells (such as muscle cells and epidermal cells) after injection204,205. Once escaping from the lysosome to the cytosol, the mRNA is sensed by Toll-like receptors (TLRs), promoting the release of type I interferon (IFN) into the extracellular matrix and creating an environment that favors Th1 responses, a pro-inflammatory response that leads to specific immunity7,206. Then, the released mRNA is translated into antigen proteins by ribosomes207. Antigen proteins translated from exogenous mRNA are extracellularly expressed in the secreted form and then taken up by antigen-presenting cells (APCs)192. Antigen proteins are degraded into fragments by the proteasome in APCs, followed by MHC-I presentation, activating CD8 T-cells, or undergo lysosomal degradation via various mechanisms such as autophagy and signal peptide, followed by MHC-II presentation, activating CD4 T-cells and leading to the maturation of B cells192,208.
Figure 3.
mRNA vaccinization in vivo. (1) Construction of mRNA lipid NPs, (2) endocytosis, (3) entry into lysosome, (4) escape from lysosome, (5) recognization by (TLRs, (6) translation of replicase, (7) self-amplification of mRNA, (8) translation of antigen protein, (9) secretion of antigen protein, (10) MHC-I presentation, (11) MHC-II presentation.
In addition to the characteristics of the mRNA vaccine, the injection routine and site also affect its effectiveness. Administration routes are essential to induce protein expression, frequency, and side effects209. For instance, 1) intraveous injection (iv): the acting time is short. After iv administration, the preparation is first ingested by reticuloendothelial-rich tissues (liver, spleen), then phagocytosed by mononuclear macrophages and, to a lesser extent, uptaken by lung and marrow210. The blood clearance rate is related to the sizes and charges of carriers. Larger nanoparticles are removed by the blood faster211. Moreover, iv administration would cause more side effects if toxic solvent remains in preparation. Also, the patient compliance with iv administration is poor due to the injection pain. 2) Subcutaneous injection (sc): This sc administration can directly transfect mRNA into DCs in lymph nodes212. In addition, when the particle size is > 150 nm, NPs are more likely to be phagocytosed by immune cells and reach lymph nodes. Nonetheless, sc is administered with limited doses (less than 2 mL). 3) intramuscular injection (im): the im could be dosed with a large dose of 2–5 mL, but it has special requirements on particle size and charge137. The particles with smaller particle sizes (<50 nm) and less surface charge could deliver mRNA to DCs with high efficacy. 4) Nasal administration: It has the advantages of convenient administration, high bioavailability and fast access to local lymphatic tissues213, but the dose is limited. 5) Intradermal injection (id): id can effectively induce a Th1 immune response, but it is also impossible to administer with a high dose5. Accordingly, we should select the appropriate administration route to improve mRNA delivery. Furthermore, direct injection of mRNA into secondary lymphoid tissue facilitates targeted antigen delivery to APCs without needing DCs migration. mRNA vaccines have many specific advantages over traditional vaccines: 1) mRNA theoretically meets all the genetic information requirements for encoding and expressing various proteins. The vaccine's protective efficacy can be optimized by modifying the mRNA sequence, providing a more convenient method of vaccine upgrading than other types of vaccines214,215; 2) the development of mRNA vaccines takes a short time, has a low cost, and is easy to realize industrialization. mRNA vaccines encoding different antigens generally posess similar production and purification process. Development costs could effectively be reduced by establishing a standardized operation process214,216; 3) mRNA has autoimmune properties able to activate adaptive immune responses through cytokines secreted by immune cells, while traditional vaccines require additional immune adjuvants to enhance immunity217; 4) mRNA is translated in vivo to produce therapeutic proteins with the potential for less immunogenicity and complete functionality, preventing contamination by foreign proteins and viruses214.
However, the development of mRNA vaccines is also limited due to mRNA instability, potential immunogenicity, and high in vivo instability199. The emergence of lipid carriers provides an effective solution for the in vivo delivery of mRNA vaccines. Currently, LLPs are the most widely and maturely used platform with the best clinical results. The possible reasons for LLPs to stand out from numerous lipid carriers are as follows: 1) Based on the preparation and production technology of LPs, as the developed version of LPs, LLPs have a more mature production and preparation process; 2) the materials of LLPs are relatively easy to get and are safe218. The addition of ionizable lipids enhances the encapsulation of mRNA and endosomal escape. The lipid carriers are predominantly used in preventative mRNA vaccines against infectious diseases and therapeutic mRNA vaccines against cancer219. Compared with traditional vaccines, mRNA vaccines can be developed in a shorter time to combat pandemic infectious diseases. In addition, mRNA opens up a new line for several infectious diseases where traditional vaccination approaches have failed. The mRNA vaccine offers a choice to fight against vaccine-preventable infectious diseases. So far, several mRNA vaccines against infectious diseases are under clinical trial. Moderna's cytomegalovirus vaccine has entered phase II, while Zika, influenza, and respiratory syncytial virus vaccines are in phase I220. There are also many mRNA tumor vaccines under clinical trial. Moderna's mRNA-4157 and BioNTech's BNT122 activate the immune system by expressing patient-specific tumor neoantigens221. Currently, tumor-associated antigens and tumor-specific neo-epitopes are the core targets of mRNA cancer vaccines7,198,222. Jabulowsky et al.223 prepared an RNA-lipoplex vaccine against melanoma. The vaccine allowed APC-targeting, efficient uptake and expression of encoded antigens. The vaccine is under a human Phase I/II dose-escalation Lipo-MERIT trial (NCT02410733) to evaluate the safety, tolerability and biological efficacy of RNA (LIP) immunotherapy in patients with stage IIIB/C and iv melanoma. In addition, various hybrid vectors have also been utilized to deliver mRNA vaccines, including LPNs, lipid nanoemulsions, etc. Regarding the formulation, cationic or ionizable lipids are essential components to improve the delivery efficiency of mRNA vaccines. mRNA vaccines are now available for various diseases such as infectious diseases and cancer. The clinical trial of LLPs for mRNA vaccine delivery is shown in Table 46,224.
Table 4.
Clinical trials for mRNA vaccine delivery (COVID-19 vaccines are not included).
| Drug | Disease | Administration route | Lipid-based carriers | Trial phase | Ref. |
|---|---|---|---|---|---|
| Core-g28v2 60mer and eOD-GT8 60mer | HIV | im | LLPs | Ⅰ (NCT05001373) | https://clinicaltrials.gov/ct2/show/NCT05001373 |
| CV7202 | Rabies | im | LLPs | Ⅰ (NCT03713086) | https://clinicaltrials.gov/ct2/show/NCT03713086 |
| GSK 692342 | Tuberculosis | im | LLPs | Ⅱ (NCT01669096) | https://clinicaltrials.gov/ct2/show/NCT01669096 |
| mRNA-1010 | Seasonal influenza | im | LLPs | Ⅰ/Ⅱ (NCT04956575) | https://clinicaltrials.gov/ct2/show/NCT04956575 |
| mRNA-1325 | Zika virus | – | LLPs | Ⅰ (NCT03014089) | https://clinicaltrials.gov/ct2/show/NCT03014089 |
| mRNA-1345 | Respiratory syncytial virus | im | LLPs | Ⅰ (NCT04528719) | https://clinicaltrials.gov/ct2/show/NCT04528719 |
| mRNA-1440 | Influenza H10N8 | im | LLPs | Ⅰ (NCT03076385) | https://clinicaltrials.gov/ct2/show/NCT03076385 |
| mRNA-1653 | Human metapneumovirus and human parainfluenza infection | im | LLPs | Ⅰ (NCT03392389) | https://clinicaltrials.gov/ct2/show/NCT03392389 |
| mRNA-1647 | Cytomegalovirus infection | im | LLPs | Ⅱ (NCT04232280) | https://clinicaltrials.gov/ct2/show/NCT04232280 |
| mRNA-1851 | Influenza H9N9 | im | LLPs | Ⅰ (NCT03345043) | https://clinicaltrials.gov/ct2/show/NCT03345043 |
| mRNA-1893 | Zika virus | im | LLPs | Ⅰ (NCT04064905) | https://clinicaltrials.gov/ct2/show/NCT04064905 |
| VAL-181388 | Chikungunya virus | im | LLPs | Ⅰ (NCT03325075) | https://clinicaltrials.gov/ct2/show/NCT03325075 |
| RG-SAM | Rabies | im | LLPs | Ⅰ (NCT04062669) | https://clinicaltrials.gov/ct2/show/NCT04062669 |
| BI 1361849 | Metastatic non-small cell lung cancer | intratumoral injection | LLPs | Ⅰ/Ⅱ (NCT03164772) | https://clinicaltrials.gov/ct2/show/NCT03164772 |
| BNT113 | Unresectable head and neck squamous cell carcinoma, metastatic head and neck cancer, recurrent head and neck cancer | iv | LPs | Ⅱ (NCT04534205) | https://clinicaltrials.gov/ct2/show/NCT04534205 |
| HARE-40 | Squamous cell carcinoma, head and neck neoplasm, cervical neoplasm, penile neoplasms malignant | id | LLPs | Ⅰ/Ⅱ (NCT03418480) | https://clinicaltrials.gov/ct2/show/NCT03418480 |
| Lipo-MERIT | Melanoma | iv | LLPs | Ⅰ (NCT02410733) | https://clinicaltrials.gov/ct2/show/NCT02410733 |
| mRNA-2416 | Relapsed/refractory solid tumor malignancies or lymphoma, ovarian cancer | intratumoral injection | LLPs | Ⅰ/Ⅱ (NCT03323398) | https://clinicaltrials.gov/ct2/show/NCT03323398 |
| mRNA-2752 | Relapsed/refractory solid tumor malignancies or lymphoma | intratumoral injection | LLPs | Ⅰ (NCT03739931) | https://clinicaltrials.gov/ct2/show/NCT03739931 |
| mRNA-4157 | Melanoma | im | LLPs | Ⅱ (NCT03897881) | https://clinicaltrials.gov/ct2/show/NCT03897881 |
| mRNA-4650 | Melanoma, colon cancer, gastrointestinal cancer, genitourinary cancer, hepatocellular cancer | im | LLPs | Ⅰ/Ⅱ (NCT03480152) | https://clinicaltrials.gov/ct2/show/NCT03480152 |
| mRNA-5671/V941 | Non-small-cell lung carcinoma, pancreatic neoplasms, colorectal neoplasms | im | LLPs | Ⅰ (NCT03948763) | https://clinicaltrials.gov/ct2/show/NCT03948763 |
| RNA-LPs | Adult glioblastoma | iv | LPs | Ⅰ (NCT04573140) | https://clinicaltrials.gov/ct2/show/NCT04573140 |
| RO7198457 | Advanced melanoma | iv | LLPs | Ⅱ (NCT03815058) | https://clinicaltrials.gov/ct2/show/NCT03815058 |
| RO7198457 | Melanoma, non-small-cell lung cancer, bladder Cancer, colorectal cancer, triple negative breast cancer, renal cancer, head and neck cancer and other solid Cancers | iv | LLPs | Ⅰ (NCT03289962) | https://clinicaltrials.gov/ct2/show/NCT03289962 |
| SAR441000 | Metastatic neoplasm | intratumoral injection | LLPs | Ⅰ (NCT03871348) | https://clinicaltrials.gov/ct2/show/NCT03871348 |
| TNBC-MERIT | Triple negative breast cancer | iv | LPs | Ⅰ (NCT02316457) | https://clinicaltrials.gov/ct2/show/NCT02316457 |
| W_ova1 | Ovarian cancer | iv | LPs | Ⅰ (NCT04163094) | https://clinicaltrials.gov/ct2/show/NCT04163094 |
| ARCT-810 | Ornithine transcarbamylase deficiency | iv | LLPs | Ⅰ (NCT04442347) | https://clinicaltrials.gov/ct2/show/NCT04442347 |
| Low Density Lipoprotein Receptor mRNA Exosomes | Familial hypercholesterolemia | iv | Exosomes | Ⅰ (NCT05043181) | https://clinicaltrials.gov/ct2/show/NCT05043181 |
| mRNA-3927 | Propionic acidemia | iv | LLPs | Ⅰ/Ⅱ (NCT05130437) | https://clinicaltrials.gov/ct2/show/NCT05130437 |
| UXO053 | Glycogen storage disease type III | iv | LLPs | Ⅰ/Ⅱ (NCT04990388) | https://clinicaltrials.gov/ct2/show/NCT04990388 |
| mRNA-6231 | Various autoimmune disorders | sc | LLPs | Ⅰ (NCT04916431) | https://clinicaltrials.gov/ct2/show/NCT04916431 |
‒, not applicable.
Id, intradermal injection; im, intramuscular injection; iv, intravenous injection; LPs, liposomes; LLPs, liposome-like nanoparticles; sc, subcutaneous injection.
3.2. COVID-19 mRNA vaccine based on lipid carriers
With the global epidemic of COVID-19, mRNA vaccines, as a new type of vaccine, have played a leading role in this battle. Here, we compared the two marked COVID-19 mRNA vaccines (mRNA-1273 from Moderna225 and BNT162b2 from BioNTech226) in antigen choice and LLPs design227.
By understanding the structure of SARS-CoV-2, the full-length spike glycoprotein (S protein) on the surface of SARS-CoV-2 and the receptor-binding domain (RBD) located at the C-terminal of the S1 subdomain, the subunit of S protein induces highly potent neutralizing antibodies and T cell-mediated immunity200,228,229. Therefore, mRNA encoding S protein or RBD was selected as an active ingredient for coronavirus vaccine development230,231. Both mRNA vaccines selected mRNA encoding the S protein, in which the mRNA sequence is slightly changed by two proline substitutions, enhancing the stability of the prefusion conformation of the glycoprotein232. In addition to antigen choice, the LLP design of the two mRNA vaccines is similar, containing ionizable lipid (enabling mRNA complexation and escape from lysosome), PEG-lipid (conferring more prolonged systemic circulation), DSPC and chol (helping pack the cargo into the LLPs) (Table 5)57. The molar ratios of the ionizable lipid: PEG-lipid: DSPC: chol are 50: 1.5: 10: 38.5 for the mRNA-1273 vaccine and 46.3: 1.6: 9.4: 42.7 for the BNT162b1 vaccine57. The ionizable lipids in the two vaccines, SM-102 (mRNA-1273) and ALC-0315 (BNT162b2), are chemically comparable. Both have branched hydrocarbon chains, optimizing the formation of nonlamellar phases and enhancing the efficiency of mRNA delivery233. Furthermore, compared to conventional ionizable lipids, ester-linkages are introduced to SM-102 and ALC-0315, improving their biodegradability218. Besides, the two vaccines include similar PEG-lipids (PEG2000-DMG and ALC-0159) and identical helper lipids (DSPC and chol)218.
Table 5.
Lipid composition of the LLP carriers of the COVID-19 mRNA vaccines.
| Vaccine name (company) | Lipids | Abbreviation or lab code | Role |
|---|---|---|---|
| mRNA-1273 (Moderna) | Heptadecan-9-yl 8-((2-hydroxyethyl) (6-oxo-6-(undecyloxy)hexyl)amino)octanoate | SM-102 | Ionizable lipid |
| 1,2-Dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 | PEG2000-DMG | PEG-lipid | |
| 1,2-Distearoyl-sn-glycero-3-phosphocholine | DSPC | Helper lipid | |
| Cholesterol | Chol | Helper lipid | |
| BNT162b2 (Pfizer/BioNTech) | (4-Hydroxybutyl)azanediyl bis(hexane-6,1-diyl)bis(2-hexyldecanoate) | ALC-0315 | Ionizable lipid |
| (2-Hexyldecanoate),2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide | ALC-0159 | PEG-lipid | |
| 1,2-Distearoyl-sn-glycero-3-phosphocholine | DSPC | Helper lipid | |
| Cholesterol | Chol | Helper lipid |
In summary, the two mRNA vaccines share many similarities. Both choose LLPs to deliver mRNA encoding the S protein with the same mRNA-to-lipid ratio, 0.05 (wt/wt)218. According to some review materials authorized by the FDA, the effectiveness of the two vaccines is comparable (mRNA-1273 is 94.1% and BNT162b2 is 95.0%). However, the variance in composition causes differences in administration and storage conditions. Regarding administration, BNT162b2 needs thawing followed by dilution with saline, while mRNA-1273 can be injected after thawing192. In terms of storage conditions, BNT162b2 is stored at −60 to −80 °C, whereas mRNA-1273 can be stored at −15 to −25 °C192. Compared with other vaccines just stored at 2–8 °C, this cryopreservation at low or even ultra-low temperatures impedes vaccine transport, storage and distribution233. For the improvement of mRNA-LLPs’ stability, the mRNA nucleotide composition should be optimized primarily. A report indicated that the introduction of modified uridines inside the mRNA could minimize the recognition of mRNA by cells and thus reduce the natural immune response induced by foreign mRNA193. In contrast, the mRNA biostability was elevated. Secondly, a better understanding of the environment of mRNA in the core of LLPs helps adjust the structure of LLPs reasonably to maintain the integrity of mRNA. In addition, more mature and accurate preparation techniques, such as lyophilization, remain to be explored.
Globally, various kinds of COVID-19 vaccines have been on the market, including mRNA vaccines, recombinant protein vaccines, recombinant adenovirus vector vaccines, and inactivated vaccines234. Among them, the clinical data on the protective efficacy of mRNA vaccines has excellent performance. The protection against mutant strains was also significantly improved after the acupuncture was strengthened. mRNA-1273 and BNT162b1 vaccines have been approved in the United States and the European Union. So far, none of the mRNA-based vaccines have been approved for marketing in China, but the development of mRNA vaccines has achieved remarkable progress recently. Several mRNA vaccines have entered the clinical trial (Table 6).
Table 6.
Clinical trials of the lipid-based mRNA vaccines for COVID-19.
| Vaccine name (company) | Lipid-based carrier | Registration number/phase |
|---|---|---|
| ChulaCov19 (Chulalongkon University) | LLPs | NCT04566276/phase I/II |
| CoV2 SAM (LNP) (GlaxoSmithKline) | LLPs | NCT04758962/phase I |
| SAM-LNP-S (Gritstone) | LLPs | NCT04776317/phase I |
| ARCT-021 (Arcturus) | LLPs | NCT04480957/phase I/II |
| LNP-nCoVsaRNA (Imperial College London) | LLPs | ISRCTN17072692/phase I |
| LNP-nCOV saRNA-02 (MRC/UVRI and LSHTM Uganda Research) | LLPs | NCT04934111/phase I |
| ARCoVax (Abogen/Walvax) | LLPs | ChiCTR2000039212/phase I/II/Ⅲ |
| LVRNA009 (Aimbio) | LLPs | ChiCTR2200057782/phase I/II |
| SW0123 (Stemirna) | LPR | ChiCTR2100047917/phase I |
| COVID-19 mRNA vaccine (RiboBio/AgnaBio) | LLPs | Phase I |
| CvnCoV (CureVac AG) | LLPs | NCT04674189/phaseⅢ |
| SYS6006 (CSPC) | LLPs | Preclinical |
| COVID-19 mRNA vaccine (CanSino Biologics) | LLPs | Preclinical |
| RQ3013 (RNAcure) | LLPs | Preclinical |
| R520A (Recbio) | LLPs | Preclinical |
LLPs, liposome-like nanoparticles; LPR, lippolyplex.
4. Summary and prospect
The most extensively considered RNA drugs are divided into two categories according to their molecular weight, siRNA and mRNA. siRNA has short sequences and a molecular weight ranging from thousands to tens of thousands of daltons, which can easily be synthesized by chemical preparation in vitro and then act using enzymes in the cell235. siRNA has been approved for disease therapy via gene silencing. These double-stranded RNAs with a molecular weight of approximately 13 kDa can repress protein translation by recruiting the RNA-induced silencing complex (RISC) to the mRNA through base pairing235. siRNA-lipid NPs have been approved by the FDA or the European Medicines Agency (EMA), including patisiran for treating hereditary transthyretin-mediated amyloidosis (hATTR)236, givosiran for treating acute hepatic porphyria237, lumasiran for treating primary hyperoxaluria type 1238, and inclisiran for treating hypercholesterolemia239. As a therapeutic drug, siRNA works by suppressing the translation of target genes or degrading mRNA to reduce abnormal-protein production, mainly at the post-transcriptional and translational levels240. MicroRNAs (miRNAs) act similarly. However, miRNA is an inherent component of intracellular RNA with a single-stranded RNA, whereas siRNA is double-stranded RNA. Due to the incomplete complementarity of miRNA, miRNA mainly acts on the 3ʹUTR of mRNA to regulate mRNA, while siRNA can work on any position of mRNA. Regarding the action mode, miRNA works by inhibiting the translation of the target gene or degrading the target gene, performing at the post-transcriptional and translational levels; in contrast, siRNA modulates the post-transcription by discomposing the target gene241.
Unlike siRNA or miRNA, mRNAs can be directly translated into target proteins in cells, such as gene editing-related CRISPR proteins, SARS-CoV-2, etc. mRNA has shown therapeutic potential in various applications, including viral vaccines, protein replacement therapies, and cancer immunotherapies54. For therapeutic-effect achievement, mRNA molecules must reach specific target cells and produce sufficient proteins of interest. mRNA can be used for precise and personalized therapy, enabling patients to produce therapeutic proteins in their bodies without struggling with the associated manufacturing problems of recombinant proteins. mRNA production is more cost-effective, faster, and more flexible than current treatments because it can be quickly produced by in vitro transcription. Whereas mRNA has a longer sequence and higher molecular weight changing from the hundreds of thousands to millions of daltons compared with siRNA or miRNA235. Therefore, mRNA does not possess the advantages of a shorter chemical synthesis cycle, flexible modification sites, and high yields like siRNA and miRNA. While the toxicity and activity of RNAi depend significantly on the present or absent sequences in the transcriptome of animal models. siRNA that works well at silencing mouse genes may have little activity against human genes242. Meanwhile, after reaching the cytoplasm, siRNA must be integrated into RISC to trigger RNAi. This process demands that the most unstable siRNA strand of 5′end hybridization is specially loaded into RISC while the other strand is degraded. Therefore, attaching the delivery material to the 5′ end of the antisense strand in siRNA delivery should be prevented. siRNA-backbone modifications and siRNA sequences must be selected carefully to guarantee that RISC accurately targets the strands and avoid hybridization to non-target mRNA moieties, which can lead to off-target gene silencing243. In contrast, mRNA therapy just needs a translation to produce a specific target protein, without considering the off-target effects. In addition, mRNA vaccines allow the rapid development of personalized medicines based on sequencing results and/or personalized conditions244. However, key issues must be addressed, including stability, immunogenicity, translation efficiency, and intracellular delivery, to elevate mRNA effectiveness244. Besides, the drawback is that mRNA only offers a short duration of protein expression and short-term transient gene expression, limiting its therapeutic use. Co-delivery of mRNA and other nucleic acid has the potential to address this issue. For instance, the combination of mRNA and DNA can integrate their properties into one system. mRNA-mediated protein expression starts quickly but lasts for a limited time, while DNA transfection has higher and sustained protein expression but starts later245. In addition, the emergence of a new generation of mRNA vaccines, saRNA, which can generate consistent and robust immune responses at low doses, marks mRNA development246. Nevertheless, improving the efficacy of the endosomal escape and intracellular delivery is still the main interest of carrier-mediated mRNA delivery.
Lipid carriers play an essential role in mRNA delivery, along with a few lipid carriers approved for clinical use247. However, the in vivo fate of lipid carrier is poorly demonstrated, compromising the formulation design efficacy248. When entering the body, mRNA lipid NPs undergo biological processes, including absorption, distribution, metabolism, and excretion. The size and surface charge significantly impact the absorption of mRNA lipid NPs. Particles smaller than 10 nm may escape from the loose lymphatic vessels and accumulate in the spleen249, while particles larger than 100 nm are difficult to diffuse into lymphatic capillaries250. So, particles having a size of 10–100 nm are more efficient in targeting lymph nodes251,252. In addition, the components, such as collagen fibers and glycosaminoglycan, in the lymph node are negatively charged; thus, neutrally or negatively charged mRNA NPs are always utilized for lymphatic delivery251. mRNA lipid-NPs have limited treatment potential due to liver accumulation. Recent findings displayed that mRNA lipid-NPs decorated with organ-selective protein could deliver drugs beyond the liver, predominantly depending on the protein properties253. mRNA lipid-NPs adsorb serum proteins in the blood and are recognized by receptors on targeting organs253. Hence, mRNA lipid-NPs could be designed to target specific organs such as the lung and spleen. Metabolism and elimination are also critical steps in the fate of mRNA lipid-NPs in vivo. High biodegradability is critical and required to ensure their safety while using. Maier et al.254 reported a novel ionizable lipid, DLin-MC3-DMA5, by introducing ester bonds into lipids. In vivo experiments showed that DLin-MC3-DMA5 was rapidly metabolized and eliminated by urine and feces in 72 h after being intravenously administered to mice.
Furthermore, the administration route significantly impacts their in vivo fate. Im and iv are the most widely used administration routes for mRNA lipid NPs delivery (Table 4). For the im administration, the lipid NPs go through the epidermis and subcutaneous tissues and reach the deep muscle tissues54. Muscle tissues have rich lymph vessels with high permeability. Therefore, mRNA lipid-NPs primarily diffuse into lymphatic circulation and then distribute to lymph nodes255. Besides the size and charge mentioned above, the ligand modification affects the targeting ability of mRNA lipid-NPs and distribution efficiency. Zhu et al.256 developed a lipid-based nanovaccine modified with mannose to target mannose receptors on APCs. Mannose-decorated NPs demonstrated prolonged retention time at the injection site, which could be detected at 96 h after im injection to mice, and increased distribution to inguinal lymph nodes compared to the non-modification nanovaccine. The results indicated that mannose modification considerably enhanced the transport of lipid NPs in lymph nodes after im dosing256. Recent studies discovered that the deformability of lipid NPs may affect the antigen transport after im injection. Song et al.257 reported a 330-nm nanoemulsion composed of albumin, squalene, and lipopeptide ovalbumin. Due to the highly flexible deformability, the NPs could adjust the size to cross the endothelial gaps (20–100 nm), leading to higher lymph-node transfer compared to solid albumin particles257. Additionally, tracing lipid components indicated that NPs were metabolized in the liver and spleen after im administration76. A further parallel study of mRNA distribution should be conducted to confirm the finding40. For iv injection, the lipid carriers enter the blood and are quickly taken up by the reticuloendothelial system (RES)210. The size and charge are the most important factors affecting the in vivo fate of lipid NPs. NPs with a size over 100 nm are primarily retained in the liver Kupffer cells or trapped in the lung1. Cationic lipid NPs are easily captured by serum protein. PEG modification is promising to compromise the interaction between lipid NPs and serum protein and extend the circulation time1. Also, the modification of lipid NPs could alter the biodistribution. Perche et al.258 decorated lipid-based formulation with glycomimetics, named Man11-LPR100, to improve the transfer efficiency of spleen DCs. The results revealed that mice injected with a green fluorescent protein (EGFP) mRNA-loaded Man11-LPR100 possessed four times DC-expressing EGFP than those injected with sugar-free LPR100258. However, liver accumulation remains the major challenge for disease therapy. The liver biodistribution of LPR100 or Man11-LPR100 is over 80%, while merely 8% NPs accumulated in the spleen, owing to the interactions between positive charge in LPR and serum proteins and erythrocytes in the liver. Strategies such as using a glycolipid containing a PEG spacer between the mannose residue and the lipid moiety could decrease the LPR's positive charge and reduce the liver's capture258. Regardless of the im or iv administration, the development of lipid NPs for mRNA delivery remains challenging, including liver accumulation, mRNA internalization and intracellular trafficking1, etc. Undoubtedly, a deeper understanding of the fate of mRNA lipid-NPs in vivo benefits lipid carriers' contribution to mRNA delivery.
Despite the approval of ionizable lipids, several challenges still need to be addressed. First, ionizable lipids in LLPs often cause acute immune responses, such as complement activation-related pseudoallergy (CARPA), an acute immunological response that can lead to anaphylactic-like shock259 and long-term toxicity in other mRNA therapy applications260. Although degradable ionizable lipids can alleviate the unwanted effects, long-term treatment still requires premedication with glucocorticoids and antihistamines261. Consequently, amino lipid optimization is required260. By optimizing the lipid tail and introducing a primary lipid (similar to that observed with an MC3-based series), Sabnis et al.90 present amino lipids with good pharmacokinetics. The lipids demonstrated little adverse effect in a one-month toxicological evaluation, providing the potential for long-term administration without activating the immune system90. In addition, Chen et al.262 formulated dexamethasone-lipid prodrug into LLPs, suppressing the production of pro-inflammatory cytokines such as TNFα, IL-1β, and IL-6. This study offers an appropriate means to prevent immune stimulation initiated by dosing mRNA formulations. Second, ionizable lipid-mediated extra hepatic delivery is another challenge for mRNA application. Many ester-based ionizable lipids could transfect the liver; therefore, the proper design of ionizable lipids with targeted ability is expected to improve non-liver drug delivery. For instance, a neurotransmitter-derived ionizable lipid exhibits a transporting ability to cross the blood–brain barrier (BBB). This brain delivery platform allowed mice brain delivery of amphotericin B (AmB), antisense oligonucleotides (ASOs) against tau and genome-editing fusion protein (−27) GFP-Cre recombinase via iv injection263. The results demonstrated that the vectors based on these derived ionizable lipids have great potential for brain delivery and treating central nervous system diseases. Furthermore, protein libraries such as phage display and bar-coding assays have been instrumental in finding proteins with affinity to other organs (tissues). These efforts are expected to reveal effective targeting ligands and thereby improve the targeting capability of LLPs beyond the liver. Third, the synthesis of ionizable lipids is cumbersome, causing scalable manufacturing challenges. E.g., synthesizing MC3 requires four steps and one week of intensive labor77. This problem might be addressed using combinatorial chemistry for simplified synthesis. As a consequence, the optimization and innovation of ionizable lipids are still highly required for mRNA delivery.
To sum up, additional efforts should be involved to get efficient vectors for mRNA delivery. As our best knowledge goes, the effectiveness of mRNA delivery lies in the following aspects: 1) mRNA/carriers complexation efficacy determines the degradation of mRNA; 2) the moderate binding strength enables the delivery system to release mRNA at the target destination; 3) efficient cellular uptake and endosomal escape18. Therefore, lipid carriers possessing these criteria could effectively deliver mRNA.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. 81872823, 82073782), the Double First-Class (CPU2018PZQ13, China) of the CPU, the Shanghai Science and Technology Committee (19430741500, China), Natural Science Foundation of the Jiangsu Higher Education Institutions of China (21KJA320003), and the Key Laboratory of Modern Chinese Medicine Preparation of Ministry of Education of Jiangxi University of Traditional Chinese Medicine (zdsys-202103).
Author contributions
Wanting Zhang, Yuxin Jiang and Yonglong He were responsible for data collection and writing the manuscript. Hamza Boucetta, Jun Wu and Zhongjian Chen participated in correction. Wei He supervised and revised the manuscript. All of the authors have read and approved the final manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2022.11.026.
Contributor Information
Jun Wu, Email: wujun9989@njum.edu.cn.
Zhongjian Chen, Email: aajian818@163.com.
Wei He, Email: weihe@cpu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Midoux P., Pichon C. Lipid-based mRNA vaccine delivery systems. Expert Rev Vaccines. 2015;14:221–234. doi: 10.1586/14760584.2015.986104. [DOI] [PubMed] [Google Scholar]
- 2.Viana I.M.O., Roussel S., Defrêne J., Lima E.M., Barabé F., Bertrand N. Innate and adaptive immune responses toward nanomedicines. Acta Pharm Sin B. 2021;11:852–870. doi: 10.1016/j.apsb.2021.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchida S., Perche F., Pichon C., Cabral H. Nanomedicine-based approaches for mRNA delivery. Mol Pharm. 2020;17:3654–3684. doi: 10.1021/acs.molpharmaceut.0c00618. [DOI] [PubMed] [Google Scholar]
- 4.Qin S.G., Tang X.S., Chen Y., Chen K., Fan N., Xiao W., et al. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct Targeted Ther. 2022;7:166. doi: 10.1038/s41392-022-01007-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichmuth A.M., Oberli M.A., Jaklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahin U., Kariko K., Tureci O. mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 7.He Q., Gao H., Tan D., Zhang H., Wang J.Z. mRNA cancer vaccines: advances, trends and challenges. Acta Pharm Sin B. 2022;12:2969–2989. doi: 10.1016/j.apsb.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richner J.M., Himansu S., Dowd K.A., Butler S.L., Salazar V., Fox J.M., et al. Modified mRNA vaccines protect against Zika virus infection. Cell. 2017;168:1114–1125.e10. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinhard K., Rengstl B., Oehm P., Michel K., Billmeier A., Hayduk N., et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science. 2020;367:446–453. doi: 10.1126/science.aay5967. [DOI] [PubMed] [Google Scholar]
- 10.Anttila V., Saraste A., Knuuti J., Jaakkola P., Hedman M., Svedlund S., et al. Synthetic mRNA encoding VEGF-A in patients undergoing coronary artery bypass grafting: design of a phase 2a clinical trial. Mol Ther Methods Clin Dev. 2020;18:464–472. doi: 10.1016/j.omtm.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gan L.M., Lagerström-Fermér M., Carlsson L.G., Arfvidsson C., Egnell A.C., Rudvik A., et al. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat Commun. 2019;10:871. doi: 10.1038/s41467-019-08852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martini P.G.V., Guey L.T. A new era for rare genetic diseases: messenger RNA therapy. Hum Gene Ther. 2019;30:1180–1189. doi: 10.1089/hum.2019.090. [DOI] [PubMed] [Google Scholar]
- 13.An D., Schneller J.L., Frassetto A., Liang S., Zhu X., Park J.S., et al. Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 2017;21:3548–3558. doi: 10.1016/j.celrep.2017.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajj K.A., Whitehead K.A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat Rev Mater. 2017;2:1–17. [Google Scholar]
- 15.Loughrey D., Dahlman J.E. Non-liver mRNA delivery. Acc Chem Res. 2022;55:13–23. doi: 10.1021/acs.accounts.1c00601. [DOI] [PubMed] [Google Scholar]
- 16.Collén A., Bergenhem N., Carlsson L., Chien K.R., Hoge S., Gan L.M., et al. VEGFA mRNA for regenerative treatment of heart failure. Nat Rev Drug Discov. 2022;21:79–80. doi: 10.1038/s41573-021-00355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoulikha M., Huang F.F., Wu Z.F., He W. COVID-19 inflammation and implications in drug delivery. J Control Release. 2022;346:260–274. doi: 10.1016/j.jconrel.2022.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan S., Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017;24:133–143. doi: 10.1038/gt.2017.5. [DOI] [PubMed] [Google Scholar]
- 19.Shang H.T., Feng X.C., Xu P.J., Wang Y., Fu H., Li Y.P. Research development of mRNA vaccine delivery based on lipid carrier. Drug Evaluation Research. 2021;44:869–875. [Google Scholar]
- 20.Mahdinloo S., Kiaie S.H., Amiri A., Hemmati S., Valizadeh H., Zakeri-Milani P. Efficient drug and gene delivery to liver fibrosis: rationale, recent advances, and perspectives. Acta Pharm Sin B. 2020;10:1279–1293. doi: 10.1016/j.apsb.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canton I., Battaglia G. Endocytosis at the nanoscale. Chem Soc Rev. 2012;41:2718–2739. doi: 10.1039/c2cs15309b. [DOI] [PubMed] [Google Scholar]
- 22.Sahay G., Alakhova D.Y., Kabanov A.V. Endocytosis of nanomedicines. J Control Release. 2010;145:182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van de Vyver T., De Smedt S.C., Raemdonck K. Modulating intracellular pathways to improve non-viral delivery of RNA therapeutics. Adv Drug Deliv Rev. 2022;181 doi: 10.1016/j.addr.2021.114041. [DOI] [PubMed] [Google Scholar]
- 24.Martens T.F., Remaut K., Demeester J., De Smedt S.C., Braeckmans K.J.N.T. Intracellular delivery of nanomaterials: how to catch endosomal escape in the act. Nano Today. 2014;9:344–364. [Google Scholar]
- 25.Agier J., Żelechowska P., Kozłowska E., Brzezińska-Błaszczyk E. Expression of surface and intracellular Toll-like receptors by mature mast cells. Cent Eur J Immunol. 2016;41:333–338. doi: 10.5114/ceji.2016.65131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Yu C. Emerging concepts of nanobiotechnology in mRNA delivery. Angew Chem Int Ed Engl. 2020;59:23374–23385. doi: 10.1002/anie.202003545. [DOI] [PubMed] [Google Scholar]
- 27.Van Tendeloo V.F., Snoeck H.W., Lardon F., Vanham G.L., Nijs G., Lenjou M., et al. Nonviral transfection of distinct types of human dendritic cells: high-efficiency gene transfer by electroporation into hematopoietic progenitor - but not monocyte-derived dendritic cells. Gene Ther. 1998;5:700–707. doi: 10.1038/sj.gt.3300626. [DOI] [PubMed] [Google Scholar]
- 28.Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–360. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- 29.Bessis N., GarciaCozar F.J., Boissier M.C. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11(Suppl 1):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 30.Bouard D., Alazard-Dany D., Cosset F.L. Viral vectors: from virology to transgene expression. Br J Pharmacol. 2009;157:153–165. doi: 10.1038/bjp.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baum C., Kustikova O., Modlich U., Li Z., Fehse B. Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum Gene Ther. 2006;17:253–263. doi: 10.1089/hum.2006.17.253. [DOI] [PubMed] [Google Scholar]
- 32.Davis M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 33.Lee H., Lytton-Jean A.K., Chen Y., Love K.T., Park A.I., Karagiannis E.D., et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol. 2012;7:389–393. doi: 10.1038/nnano.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du X.Q., Hou Y.Q., Huang J., Pang Y., Ruan C.L., Wu W., et al. Cytosolic delivery of the immunological adjuvant Poly I:C and cytotoxic drug crystals via a carrier-free strategy significantly amplifies immune response. Acta Pharm Sin B. 2021;11:3272–3285. doi: 10.1016/j.apsb.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pack D.W., Hoffman A.S., Pun S., Stayton P.S. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 36.Sokolova V., Epple M. Inorganic nanoparticles as carriers of nucleic acids into cells. Angew Chem Int Ed Engl. 2008;47:1382–1395. doi: 10.1002/anie.200703039. [DOI] [PubMed] [Google Scholar]
- 37.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 38.Selby L.I., Cortez-Jugo C.M., Such G.K., Johnston A.P.R. Nanoescapology: progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:e1452. doi: 10.1002/wnan.1452. [DOI] [PubMed] [Google Scholar]
- 39.Gregoriadis G. Liposomes in drug delivery: how it all happened. Pharmaceutics. 2016;8:19. doi: 10.3390/pharmaceutics8020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J., Kim D., Byun J., Wu Y., Park J., Oh Y.K. In vivo fate and intracellular trafficking of vaccine delivery systems. Adv Drug Deliv Rev. 2022;186 doi: 10.1016/j.addr.2022.114325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thapa Magar K., Boafo G.F., Li X., Chen Z., He W. Liposome-based delivery of biological drugs. Chin Chem Lett. 2022;33:587–596. [Google Scholar]
- 42.Zhang C., Ma Y., Zhang J., Kuo J.C., Zhang Z., Xie H., et al. Modification of lipid-based nanoparticles: an efficient delivery system for nucleic acid-based immunotherapy. Molecules. 2022;27:1943. doi: 10.3390/molecules27061943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu F.Q., Jiang S.P., Du Y.Z., Yuan H., Ye Y.Q., Zeng S. Preparation and characteristics of monostearin nanostructured lipid carriers. Int J Pharm. 2006;314:83–89. doi: 10.1016/j.ijpharm.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 44.Zoulikha M., Xiao Q.Q., Boafo G.F., Sallam M.A., Chen Z.J., He W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm Sin B. 2022;12:600–620. doi: 10.1016/j.apsb.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao Q.Q., Li X.T., Liu C., Yang Y., Hou Y.Q., Wang Y., et al. Liposome-based anchoring and core-encapsulation for combinatorial cancer therapy. Chin Chem Lett. 2022;33:4191–4196. [Google Scholar]
- 46.Bangham A.D., Horne R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1964;8:660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 47.Bulbake U., Doppalapudi S., Kommineni N., Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9:12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao Q.Q., Li X.T., Liu C., Jiang Y.X., He Y.L., Zhang W.T., et al. Improving cancer immunotherapy via co-delivering checkpoint blockade and thrombospondin-1 downregulator. Acta Pharm Sin B. 2022 doi: 10.1016/j.apsb.2022.07.012. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barenholz Y. Doxil®--the first FDA-approved nano-drug:lessons learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 50.Rizzardinl G., Pastecchia C., Vigevanl G.M., Miiella A.M. Stealth liposomal doxorubicin or bleomycin/vincristine for the treatment of AIDS-related Kaposi's sarcoma: 17. J Acquir Immune Defic Syndr. 1997;14:A20. [Google Scholar]
- 51.Neale M.H., Lamont A., Hindley A., Kurbacher C.M., Cree I.A. The ex vivo effect of high concentrations of doxorubicin on recurrent ovarian carcinoma. Anti Cancer Drugs. 2000;11:865–871. doi: 10.1097/00001813-200011000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Torchilin V.P. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 53.Buschmann M.D., Carrasco M.J., Alishetty S., Paige M., Alameh M.G., Weissman D. Nanomaterial delivery systems for mRNA vaccines. Vaccines (Basel) 2021;9:65. doi: 10.3390/vaccines9010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hou X.C., Zaks T., Langer R., Dong Y.Z. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinon F., Krishnan S., Lenzen G., Magné R., Gomard E., Guillet J.G., et al. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur J Immunol. 1993;23:1719–1722. doi: 10.1002/eji.1830230749. [DOI] [PubMed] [Google Scholar]
- 56.Li W., Szoka F.C., Jr. Lipid-based nanoparticles for nucleic acid delivery. Pharm Res (N Y) 2007;24:438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 57.Tenchov R., Bird R., Curtze A.E., Zhou Q.Q. Lipid nanoparticles-from liposomes to mRNA vaccine delivery, a landscape of research diversity and ddvancement. ACS Nano. 2021;15:16982–17015. doi: 10.1021/acsnano.1c04996. [DOI] [PubMed] [Google Scholar]
- 58.Behr M., Zhou J., Xu B., Zhang H.W. In vivo delivery of CRISPR-Cas9 therapeutics: progress and challenges. Acta Pharm Sin B. 2021;11:2150–2171. doi: 10.1016/j.apsb.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malone R.W., Felgner P.L., Verma I.M. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci U S A. 1989;86:6077–6081. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hung H.Y., Miao M.S., Zhu Y.J., Yang J., Wang S.Q. mRNA vaccine delivery system based on liposome: research advances. J Int Pharm Res. 2019;46:339–346. [Google Scholar]
- 61.Krienke C., Kolb L., Diken E., Streuber M., Kirchhoff S., Bukur T., et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371:145–153. doi: 10.1126/science.aay3638. [DOI] [PubMed] [Google Scholar]
- 62.Zohra F.T., Chowdhury E.H., Nagaoka M., Akaike T. Drastic effect of nanoapatite particles on liposome-mediated mRNA delivery to mammalian cells. Anal Biochem. 2005;345:164–166. doi: 10.1016/j.ab.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 63.Zhang R., Tang L., Tian Y., Ji X., Hu Q.Y., Zhou B.L., et al. DP7-C-modified liposomes enhance immune responses and the antitumor effect of a neoantigen-based mRNA vaccine. J Control Release. 2020;328:210–221. doi: 10.1016/j.jconrel.2020.08.023. [DOI] [PubMed] [Google Scholar]
- 64.Zhang R., Tang L., Tian Y., Ji X., Hu Q.Y., Zhou B.L., et al. Cholesterol-modified DP7 enhances the effect of individualized cancer immunotherapy based on neoantigens. Biomaterials. 2020;241 doi: 10.1016/j.biomaterials.2020.119852. [DOI] [PubMed] [Google Scholar]
- 65.Zhang X.Y., Men K., Zhang Y.F., Zhang R., Yang L., Duan X.M. Local and systemic delivery of mRNA encoding survivin-T34A by lipoplex for efficient colon cancer gene therapy. Int J Nanomed. 2019;14:2733–2751. doi: 10.2147/IJN.S198747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamada Y., Sato Y., Nakamura T., Harashima H. Innovative cancer nanomedicine based on immunology, gene editing, intracellular trafficking control. J Control Release. 2022;348:357–369. doi: 10.1016/j.jconrel.2022.05.033. [DOI] [PubMed] [Google Scholar]
- 67.Lv H.T., Zhang S., B Wang B., Cui S.H., Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 68.Lokugamage M.P., Vanover D., Beyersdorf J., Hatit M.Z.C., Rotolo L., Echeverri E.S., et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat Biomed Eng. 2021;5:1059–1068. doi: 10.1038/s41551-021-00786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shan X.T., Gong X., Li J., Wen J.Y., Li Y., Zhang Z.W. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm Sin B. 2022;12:3028–3048. doi: 10.1016/j.apsb.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yanez Arteta M., Kjellman T., Bartesaghi S., Wallin S., Wu X., Kvist A.J., et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc Natl Acad Sci U S A. 2018;115:E3351–E3360. doi: 10.1073/pnas.1720542115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zuo Q.T., Xiong W.B. Progress of mRNA vaccine delivery system based on non-viral vector. Sichuan J Physiol Sci. 2020;42:370–374. [Google Scholar]
- 72.Hajj K.A., Ball R.L., Deluty S.B., Singh S.R., Strelkova D., Knapp C.M., et al. Branched-tail lipid nanoparticles potently deliver mRNA in vivo due to enhanced ionization at endosomal pH. Small. 2019;15 doi: 10.1002/smll.201805097. [DOI] [PubMed] [Google Scholar]
- 73.Paramasivam P., Franke C., Stöter M., Höijer A., Bartesaghi S., Sabirsh A., et al. Endosomal escape of delivered mRNA from endosomal recycling tubules visualized at the nanoscale. J Cell Biol. 2022;221 doi: 10.1083/jcb.202110137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hafez I.M., Maurer N., Cullis P.R. On the mechanism whereby cationic lipids promote intracellular delivery of polynuclear acids. Gene Ther. 2001;8:1188–1196. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- 75.Alameh M.G., Tombacz I., Bettini E., Lederer K., Sittplangkoon C., Wilmore J.R., et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2021;54:2877–2892. doi: 10.1016/j.immuni.2021.11.001. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hassett K.J., Benenato K.E., Jacquinet E., Lee A., Woods A., Yuzhakov O., et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol Ther Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jayaraman M., Ansell S.M., Mui B.L., Tam Y.K., Chen J.X., Du X.Y., et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing. in vivo. Angew Chem Int Ed Engl. 2012;51:8529–8533. doi: 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Algarni A., Pilkington E.H., Suys E.J.A., Al-Wassiti H., Pouton C.W., Truong N.P. In vivo delivery of plasmid DNA by lipid nanoparticles: the influence of ionizable cationic lipids on organ-selective gene expression. Biomater Sci. 2022;10:2940–2952. doi: 10.1039/d2bm00168c. [DOI] [PubMed] [Google Scholar]
- 79.Semple S.C., Akinc A., Chen J., Sandhu A.P., Mui B.L., Cho C.K., et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 80.Lin P.J., Tam Y.Y., Hafez I., Sandhu A., Chen S., Ciufolini M.A., et al. Influence of cationic lipid composition on uptake and intracellular processing of lipid nanoparticle formulations of siRNA. Nanomedicine. 2013;9:233–246. doi: 10.1016/j.nano.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 81.Sahay G., Querbes W., Alabi C., Eltoukhy A., Sarkar S., Zurenko C., et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol. 2013;31:653–658. doi: 10.1038/nbt.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whitehead K.A., Dorkin J.R., Vegas A.J., Chang P.H., Veiseh O., Matthews J., et al. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat Commun. 2014;5:4277. doi: 10.1038/ncomms5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang W., Feng S., Ye Z.Y., Gao H.L., Lin J.Z., Ouyang D. Prediction of lipid nanoparticles for mRNA vaccines by the machine learning algorithm. Acta Pharm Sin B. 2022;12:2950–2962. doi: 10.1016/j.apsb.2021.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holland J.W., Hui C., Cullis P.R., Madden T.D. Poly(ethylene glycol)--lipid conjugates regulate the calcium-induced fusion of liposomes composed of phosphatidylethanolamine and phosphatidylserine. Biochemistry. 1996;35:2618–2624. doi: 10.1021/bi952000v. [DOI] [PubMed] [Google Scholar]
- 85.Kulkarni J.A., Witzigmann D., Leung J., Tam Y.Y.C., Cullis P.R. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale. 2019;11:21733–21739. doi: 10.1039/c9nr09347h. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki T., Suzuki Y., Hihara T., Kubara K., Kondo K., Hyodo K., et al. PEG shedding-rate-dependent blood clearance of PEGylated lipid nanoparticles in mice: faster PEG shedding attenuates anti-PEG IgM production. Int J Pharm. 2020;588 doi: 10.1016/j.ijpharm.2020.119792. [DOI] [PubMed] [Google Scholar]
- 87.Judge A., McClintock K., Phelps J.R., Maclachlan I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol Ther. 2006;13:328–337. doi: 10.1016/j.ymthe.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 88.Gabizon A., Catane R., Uziely B., Kaufman B., Safra T., Cohen R., et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54:987–992. [PubMed] [Google Scholar]
- 89.Dams E.T., Laverman P., Oyen W.J., Storm G., Scherphof G.L., van Der Meer J.W., et al. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Therapeut. 2000;292:1071–1079. [PubMed] [Google Scholar]
- 90.Sabnis S., Kumarasinghe E.S., Salerno T., Mihai C., Ketova T., Senn J.J., et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol Ther. 2018;26:1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Besin G., Milton J., Sabnis S., Howell R., Mihai C., Burke K., et al. Accelerated blood clearance of lipid nanoparticles entails a biphasic humoral response of B-1 followed by B-2 lymphocytes to distinct antigenic moieties. Immunohorizons. 2019;3:282–293. doi: 10.4049/immunohorizons.1900029. [DOI] [PubMed] [Google Scholar]
- 92.Mohamed M., Abu Lila A.S., Shimizu T., Alaaeldin E., Hussein A., Sarhan H.A., et al. PEGylated liposomes: immunological responses. Sci Technol Adv Mater. 2019;20:710–724. doi: 10.1080/14686996.2019.1627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Semple S.C., Chonn A., Cullis P.R. Influence of cholesterol on the association of plasma proteins with liposomes. Biochemistry. 1996;35:2521–2525. doi: 10.1021/bi950414i. [DOI] [PubMed] [Google Scholar]
- 94.Brader M.L., Williams S.J., Banks J.M., Hui W.H., Zhou Z.H., Jin L. Encapsulation state of messenger RNA inside lipid nanoparticles. Biophys J. 2021;120:2766–2770. doi: 10.1016/j.bpj.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sebastiani F., Yanez Arteta M., Lerche M., Porcar L., Lang C., Bragg R.A., et al. Apolipoprotein E binding drives structural and compositional rearrangement of mRNA-containing lipid nanoparticles. ACS Nano. 2021;15:6709–6722. doi: 10.1021/acsnano.0c10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Patel S., Ashwanikumar N., Robinson E., Xia Y., Mihai C., Griffith J.P., 3rd, et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat Commun. 2020;11:983. doi: 10.1038/s41467-020-14527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cullis P.R., Hope M.J. Lipid nanoparticle systems for enabling gene therapies. Mol Ther. 2017;25:1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mirchandani Y., Patravale V.B., S B Solid lipid nanoparticles for hydrophilic drugs. J Control Release. 2021;335:457–464. doi: 10.1016/j.jconrel.2021.05.032. [DOI] [PubMed] [Google Scholar]
- 99.Yaghmur A., Mu H. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharm Sin B. 2021;11:871–885. doi: 10.1016/j.apsb.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amiri M., Jafari S., Kurd M., Mohamadpour H., Khayati M., Ghobadinezhad F., et al. Engineered solid lipid nanoparticles and nanostructured lipid carriers as new generations of blood–brain barrier transmitters. ACS Chem Neurosci. 2021;12:4475–4490. doi: 10.1021/acschemneuro.1c00540. [DOI] [PubMed] [Google Scholar]
- 101.He W., Turkeshi A., Li X.T., Zhang H.W. Progress in systemic co-delivery of microRNAs and chemotherapeutics for cancer treatment by using lipid-based nanoparticles. Ther Deliv. 2020;11:591–603. doi: 10.4155/tde-2020-0052. [DOI] [PubMed] [Google Scholar]
- 102.Del Pozo-Rodriguez A., Solinis M.A., Rodriguez-Gascon A. Applications of lipid nanoparticles in gene therapy. Eur J Pharm Biopharm. 2016;109:184–193. doi: 10.1016/j.ejpb.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 103.Gomez-Aguado I., Rodriguez-Castejon J., Vicente-Pascual M., Rodriguez-Gascon A., Pozo-Rodriguez A.D., Solinis Aspiazu M.A. Nucleic acid delivery by solid lipid nanoparticles containing switchable lipids: plasmid DNA vs. messenger RNA. Molecules. 2020;25:5995. doi: 10.3390/molecules25245995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mehnert W., Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–196. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 105.Gordillo-Galeano A., Mora-Huertas C.E. Solid lipid nanoparticles and nanostructured lipid carriers: a review emphasizing on particle structure and drug release. Eur J Pharm Biopharm. 2018;133:285–308. doi: 10.1016/j.ejpb.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 106.Naseri N., Valizadeh H., Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharmaceut Bull. 2015;5:305–313. doi: 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guo Y., Bera H., Shi C.Z., Zhang L., Cun D.M., Yang M.S. Pharmaceutical strategies to extend pulmonary exposure of inhaled medicines. Acta Pharm Sin B. 2021;11:2565–2584. doi: 10.1016/j.apsb.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scioli Montoto S., Muraca G., Ruiz M.E. Solid lipid nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects. Front Mol Biosci. 2020;7 doi: 10.3389/fmolb.2020.587997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Müller R.H., Radtke M., Wissing S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54(Suppl 1):S131–S155. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 110.Beloqui A., Solinis M.A., Rodriguez-Gascon A., Almeida A.J., Preat V. Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomedicine. 2016;12:143–161. doi: 10.1016/j.nano.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 111.Gerhardt A., Voigt E., Archer M., Reed S., Larson E., Van Hoeven N., et al. A flexible, thermostable nanostructured lipid carrier platform for RNA vaccine delivery. Mol Ther Methods Clin Dev. 2022;25:205–214. doi: 10.1016/j.omtm.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Owens D.E., Peppas N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 113.Wang X.C., Mohammad I.S., Fan L.F., Zhao Z.M., Nurunnabi M., Sallam M.A., et al. Delivery strategies of amphotericin B for invasive fungal infections. Acta Pharm Sin B. 2021;11:2585–2604. doi: 10.1016/j.apsb.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang L.F., Chan J.M., Gu F.X., Rhee J.W., Wang A.Z., Radovic-Moreno A.F., et al. Self-assembled lipid--polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano. 2008;2:1696–1702. doi: 10.1021/nn800275r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meng X.J., Zhang Z., Tong J., Sun H., Fawcett J.P., Gu J.K. The biological fate of the polymer nanocarrier material monomethoxy poly(ethylene glycol)-block-poly(d,l-lactic acid) in rat. Acta Pharm Sin B. 2021;11:1003–1009. doi: 10.1016/j.apsb.2021.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Semple S.C., Chonn A., Cullis P.R. Interactions of liposomes and lipid-based carrier systems with blood proteins: relation to clearance behaviour. in vivo. Adv Drug Deliv Rev. 1998;32:3–17. doi: 10.1016/s0169-409x(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 117.Hadinoto K., Sundaresan A., Cheow W.S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm. 2013;85:427–443. doi: 10.1016/j.ejpb.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 118.Zhou Y., Fang A.P., Wang F.Z., Li H.L., Jin Q.S., Huang L.J., et al. Core-shell lipid-polymer nanoparticles as a promising ocular drug delivery system to treat glaucoma. Chin Chem Lett. 2020;31:494–500. [Google Scholar]
- 119.Zhao W.Y., Zhang C.X., Li B., Zhang X.F., Luo X., Zeng C.X., et al. Lipid polymer hybrid nanomaterials for mRNA delivery. Cell Mol Bioeng. 2018;11:397–406. doi: 10.1007/s12195-018-0536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meyer R.A., Hussmann G.P., Peterson N.C., Santos J.L., Tuesca A.D. A scalable and robust cationic lipid/polymer hybrid nanoparticle platform for mRNA delivery. Int J Pharm. 2022;611 doi: 10.1016/j.ijpharm.2021.121314. [DOI] [PubMed] [Google Scholar]
- 121.Yasar H., Biehl A., De Rossi C., Koch M., Murgia X., Loretz B., et al. Kinetics of mRNA delivery and protein translation in dendritic cells using lipid-coated PLGA nanoparticles. J Nanobiotechnol. 2018;16:72. doi: 10.1186/s12951-018-0401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Su X., Fricke J., Kavanagh D.G., Irvine D.J. In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol Pharm. 2011;8:774–787. doi: 10.1021/mp100390w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pancani E., Menendez-Miranda M., Pastor A., Brisset F., Bernet-Camard M.F., Desmaële D., et al. Nanoparticles with high payloads of pipemidic acid, a poorly soluble crystalline drug: drug-initiated polymerization and self-assembly approach. Acta Pharm Sin B. 2018;8:420–431. doi: 10.1016/j.apsb.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xue F., Li X., Qin L.X., Liu X.Y., Li C., Adhikari B. Anti-aging properties of phytoconstituents and phyto-nanoemulsions and their application in managing aging-related diseases. Adv Drug Deliv Rev. 2021;176 doi: 10.1016/j.addr.2021.113886. [DOI] [PubMed] [Google Scholar]
- 125.Taina-Gonzalez L., de la Fuente M. The potential of nanomedicine to unlock the limitless applications of mRNA. Pharmaceutics. 2022;14:460. doi: 10.3390/pharmaceutics14020460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sanchez-Lopez E., Guerra M., Dias-Ferreira J., Lopez-Machado A., Ettcheto M., Cano A., et al. Current applications of nanoemulsions in cancer therapeutics. Nanomaterials. 2019;9:821. doi: 10.3390/nano9060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.A N, Kovooru L., Behera A.K., Kumar K.P.P., Srivastava P. A critical review of synthesis procedures, applications and future potential of nanoemulsions. Adv Colloid Interface Sci. 2021;287 doi: 10.1016/j.cis.2020.102318. [DOI] [PubMed] [Google Scholar]
- 128.Costa C.P., Moreira J.N., Sousa Lobo J.M., Silva A.C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: a current overview of in vivo studies. Acta Pharm Sin B. 2021;11:925–940. doi: 10.1016/j.apsb.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gupta A., Eral H.B., Hatton T.A., Doyle P.S. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12:2826–2841. doi: 10.1039/c5sm02958a. [DOI] [PubMed] [Google Scholar]
- 130.Singh Y., Meher J.G., Raval K., Khan F.A., Chaurasia M., Jain N.K., et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 131.Teixeira H.F., Bruxel F., Fraga M., Schuh R.S., Zorzi G.K., Matte U., et al. Cationic nanoemulsions as nucleic acids delivery systems. Int J Pharm. 2017;534:356–367. doi: 10.1016/j.ijpharm.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 132.Ji G.F., Ma L.S., Yao H.C., Ma S., Si X.H., Wang Y.L., et al. Precise delivery of obeticholic acid via nanoapproach for triggering natural killer T cell-mediated liver cancer immunotherapy. Acta Pharm Sin B. 2020;10:2171–2182. doi: 10.1016/j.apsb.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fraga M., Laux M., Zandona B., Santos G.R., Giuberti C.D., de Oliveira M.C., et al. Optimization of stearylamine-based nanoemulsions obtained by spontaneous emulsification process as nucleic acids delivery systems. J Drug Deliv Sci Technol. 2008;18:398–403. [Google Scholar]
- 134.Fraga M., de Carvalho T.G., Bidone J., Schuh R.S., Matte U., Teixeira H.F. Factors influencing transfection efficiency of pIDUA/nanoemulsion complexes in a mucopolysaccharidosis type I murine model. Int J Nanomed. 2017;12:2061–2067. doi: 10.2147/IJN.S121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nagachinta S., Bouzo B.L., Vazquez-Rios A.J., Lopez R., de la Fuente M. Sphingomyelin-based nanosystems (SNs) for the development of anticancer miRNA therapeutics. Pharmaceutics. 2020;12:189. doi: 10.3390/pharmaceutics12020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pirhadi S., Amani A. Molecular dynamics simulation of siRNA loading into a nanoemulsion as a potential carrier. J Mol Model. 2020;26:215. doi: 10.1007/s00894-020-04471-9. [DOI] [PubMed] [Google Scholar]
- 137.Brito L.A., Chan M., Shaw C.A., Hekele A., Carsillo T., Schaefer M., et al. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol Ther. 2014;22:2118–2129. doi: 10.1038/mt.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O'Hagan D.T., van der Most R., Lodaya R.N., Coccia M., Lofano G. "World in motion" - emulsion adjuvants rising to meet the pandemic challenges. NPJ Vaccines. 2021;6:158. doi: 10.1038/s41541-021-00418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Del Giudice G., Rappuoli R., Didierlaurent A.M. Correlates of adjuvanticity: a review on adjuvants in licensed vaccines. Semin Immunol. 2018;39:14–21. doi: 10.1016/j.smim.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 140.Brito L.A., Kommareddy S., Maione D., Uematsu Y., Giovani C., Berlanda Scorza F., et al. Self-amplifying mRNA vaccines. Adv Genet. 2015;89:179–233. doi: 10.1016/bs.adgen.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 141.Blakney A.K., McKay P.F., Hu K., Samnuan K., Jain N., Brown A., et al. Polymeric and lipid nanoparticles for delivery of self-amplifying RNA vaccines. J Control Release. 2021;338:201–210. doi: 10.1016/j.jconrel.2021.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bogers W.M., Oostermeijer H., Mooij P., Koopman G., Verschoor E.J., Davis D., et al. Potent immune responses in rhesus macaques induced by nonviral delivery of a self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic nanoemulsion. J Infect Dis. 2015;211:947–955. doi: 10.1093/infdis/jiu522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stokes A., Pion J., Binazon O., Laffont B., Bigras M., Dubois G., et al. Nonclinical safety assessment of repeated administration and biodistribution of a novel rabies self-amplifying mRNA vaccine in rats. Regul Toxicol Pharmacol. 2020;113 doi: 10.1016/j.yrtph.2020.104648. [DOI] [PubMed] [Google Scholar]
- 144.Barile L., Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 145.Elsharkasy O.M., Nordin J.Z., Hagey D.W., de Jong O.G., Schiffelers R.M., Andaloussi S.E., et al. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–343. doi: 10.1016/j.addr.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 146.Wang T.Q., Fu Y.N., Sun S.J., Huang C.Y., Yi Y.F., Wang J.Q., et al. Exosome-based drug delivery systems in cancer therapy. Chin Chem Lett. 2022 doi: 10.1016/j.cclet.2022.05.022. Available from: [DOI] [Google Scholar]
- 147.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 148.Zhao Y., Li X.L., Zhang W.B., Yu L.L., Wang Y., Deng Z., et al. Trends in the biological functions and medical applications of extracellular vesicles and analogues. Acta Pharm Sin B. 2021;11:2114–2135. doi: 10.1016/j.apsb.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fang Z.W., Zhang X.Y., Huang H., Wu J. Exosome based miRNA delivery strategy for disease treatment. Chin Chem Lett. 2022;33:1693–1704. [Google Scholar]
- 150.Ke W., Afonin K.A. Exosomes as natural delivery carriers for programmable therapeutic nucleic acid nanoparticles (NANPs) Adv Drug Deliv Rev. 2021;176 doi: 10.1016/j.addr.2021.113835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yan Y., Du C.C., Duan X.X., Yao X.H., Wan J.J., Jiang Z.M., et al. Inhibiting collagen I production and tumor cell colonization in the lung via miR-29a-3p loading of exosome-/liposome-based nanovesicles. Acta Pharm Sin B. 2022;12:939–951. doi: 10.1016/j.apsb.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 153.de Jong O.G., Kooijmans S.A.A., Murphy D.E., Jiang L., Evers M.J.W., Sluijter J.P.G., et al. Drug delivery with extracellular vesicles: from imagination to innovation. Acc Chem Res. 2019;52:1761–1770. doi: 10.1021/acs.accounts.9b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Koh E., Lee E.J., Nam G.H., Hong Y., Cho E., Yang Y., et al. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–129. doi: 10.1016/j.biomaterials.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 155.Smyth T., Kullberg M., Malik N., Smith-Jones P., Graner M.W., Anchordoquy T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control release. 2015;199:145–155. doi: 10.1016/j.jconrel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.van Dongen H.M., Masoumi N., Witwer K.W., Pegtel D.M. Extracellular vesicles exploit viral entry routes for cargo delivery. Microbiol Mol Biol Rev. 2016;80:369–386. doi: 10.1128/MMBR.00063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Heusermann W., Hean J., Trojer D., Steib E., von Bueren S., Graff-Meyer A., et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol. 2016;213:173–184. doi: 10.1083/jcb.201506084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 159.Chen H.W., Chengalvala V., Hu H.X., Sun D.X. Tumor-derived exosomes: nanovesicles made by cancer cells to promote cancer metastasis. Acta Pharm Sin B. 2021;11:2136–2149. doi: 10.1016/j.apsb.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cooper J.M., Wiklander P.B., Nordin J.Z., Al-Shawi R., Wood M.J., Vithlani M., et al. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord. 2014;29:1476–1485. doi: 10.1002/mds.25978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tian Y.H., Li S.P., Song J., Ji T.J., Zhu M.T., Anderson G.J., et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 162.Day N.B., Wixson W.C., Shields CWt. Magnetic systems for cancer immunotherapy. Acta Pharm Sin B. 2021;11:2172–2196. doi: 10.1016/j.apsb.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim J., Afshari A., Sengupta R., Sebastiano V., Gupta A., Kim Y.H. Replication study: melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Elife. 2018;7 doi: 10.7554/eLife.39944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lai C.P., Mardini O., Ericsson M., Prabhakar S., Maguire C., Chen J.W., et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8:483–494. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schult P., Roth H., Adams R.L., Mas C., Imbert L., Orlik C., et al. microRNA-122 amplifies hepatitis C virus translation by shaping the structure of the internal ribosomal entry site. Nat Commun. 2018;9:2613. doi: 10.1038/s41467-018-05053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sun W.Q., Xing C.Y., Zhao L.B., Zhao P., Yang G.D., Yuan L.J. Ultrasound assisted exosomal delivery of tissue responsive mRNA for enhanced efficacy and minimized off-target effects. Mol Ther Nucleic Acids. 2020;20:558–567. doi: 10.1016/j.omtn.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pan D.N., Fujimoto M., Lopes A., Wang Y.X. Twist-1 is a PPARdelta-inducible, negative-feedback regulator of PGC-1 alpha in brown fat metabolism. Cell. 2009;137:73–86. doi: 10.1016/j.cell.2009.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shi C.M., Zhang M., Tong M.L., Yang L., Pang L.X., Chen L., et al. miR-148a is associated with obesity and modulates adipocyte differentiation of mesenchymal stem cells through Wnt signaling. Sci Rep. 2015;5:9930. doi: 10.1038/srep09930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang Z.G., Shi J.F., Xie J., Wang Y.F., Sun J.Y., Liu T.Z., et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4:69–83. doi: 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Aslan C., Kiaie S.H., Zolbanin N.M., Lotfinejad P., Ramezani R., Kashanchi F., et al. Exosomes for mRNA delivery: a novel biotherapeutic strategy with hurdles and hope. BMC Biotechnol. 2021;21:20. doi: 10.1186/s12896-021-00683-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu J.Y., Ren L.W., Li S., Li W., Zheng X.J., Yang Y.H., et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11:2783–2797. doi: 10.1016/j.apsb.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 173.Ramasamy I. Recent advances in physiological lipoprotein metabolism. Clin Chem Lab Med. 2014;52:1695–1727. doi: 10.1515/cclm-2013-0358. [DOI] [PubMed] [Google Scholar]
- 174.Feingold K.R. Endotext. MDText.com, Inc.; South Dartmouth (MA): 2000. Introduction to lipids and lipoproteins. PMID: 6247089. [Google Scholar]
- 175.Busatto S., Walker S.A., Grayson W., Pham A., Tian M., Nesto N., et al. Lipoprotein-based drug delivery. Adv Drug Deliv Rev. 2020;159:377–390. doi: 10.1016/j.addr.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mooberry L.K., Sabnis N.A., Panchoo M., Nagarajan B., Lacko A.G. Targeting the SR-B1 receptor as a gateway for cancer therapy and imaging. Front Pharmacol. 2016;7:466. doi: 10.3389/fphar.2016.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gallagher E.J., Zelenko Z., Neel B.A., Antoniou I.M., Rajan L., Kase N., et al. Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia. Oncogene. 2017;36:6462–6471. doi: 10.1038/onc.2017.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li X.S., Zhu R.G., Jiang H., Yin Q.W., Gu J.M., Chen J.J., et al. Autophagy enhanced by curcumin ameliorates inflammation in atherogenesis via the TFEB-P300-BRD4 axis. Acta Pharm Sin B. 2022;12:2280–2299. doi: 10.1016/j.apsb.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Busatto S., Zendrini A., Radeghieri A., Paolini L., Romano M., Presta M., et al. The nanostructured secretome. Biomater Sci. 2019;8:39–63. doi: 10.1039/c9bm01007f. [DOI] [PubMed] [Google Scholar]
- 180.Karimi N., Cvjetkovic A., Jang S.C., Crescitelli R., Hosseinpour Feizi M.A., Nieuwland R., et al. Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins. Cell Mol Life Sci. 2018;75:2873–2886. doi: 10.1007/s00018-018-2773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Suh A., Pham A., Cress M.J., Pincelli T., TerKonda S.P., Bruce A.J., et al. Adipose-derived cellular and cell-derived regenerative therapies in dermatology and aesthetic rejuvenation. Ageing Res Rev. 2019;54 doi: 10.1016/j.arr.2019.100933. [DOI] [PubMed] [Google Scholar]
- 182.Nikanjam M., Gibbs A.R., Hunt C.A., Budinger T.F., Forte T.M. Synthetic nano-LDL with paclitaxel oleate as a targeted drug delivery vehicle for glioblastoma multiforme. J Control Release. 2007;124:163–171. doi: 10.1016/j.jconrel.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 183.Kim Y., Fay F., Cormode D.P., Sanchez-Gaytan B.L., Tang J., Hennessy E.J., et al. Single step reconstitution of multifunctional high-density lipoprotein-derived nanomaterials using microfluidics. ACS Nano. 2013;7:9975–9983. doi: 10.1021/nn4039063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Thaxton C.S., Rink J.S., Naha P.C., Cormode D.P. Lipoproteins and lipoprotein mimetics for imaging and drug delivery. Adv Drug Deliv Rev. 2016;106:116–131. doi: 10.1016/j.addr.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Huang H., Cruz W., Chen J., Zheng G. Learning from biology: synthetic lipoproteins for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:298–314. doi: 10.1002/wnan.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nakayama T., Butler J.S., Sehgal A., Severgnini M., Racie T., Sharman J., et al. Harnessing a physiologic mechanism for siRNA delivery with mimetic lipoprotein particles. Mol Ther. 2012;20:1582–1589. doi: 10.1038/mt.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.McMahon K.M., Plebanek M.P., Thaxton C.S. Properties of native high-density lipoproteins inspire synthesis of actively targeted in vivo siRNA delivery vehicles. Adv Funct Mater. 2016;26:7824–7835. doi: 10.1002/adfm.201602600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Balasubramanian S., Gunasekaran K., Sasidharan S., Jeyamanickavel Mathan V., Perumal E. MicroRNAs and xenobiotic toxicity: an overview. Toxicol Rep. 2020;7:583–595. doi: 10.1016/j.toxrep.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li Y.M., Ye Z.F., Yang H.Y., Xu Q.B. Tailoring combinatorial lipid nanoparticles for intracellular delivery of nucleic acids, proteins, and drugs. Acta Pharm Sin B. 2022;12:2624–2639. doi: 10.1016/j.apsb.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.German C.A., Shapiro M.D. Small interfering RNA therapeutic inclisiran: a new approach to targeting PCSK9. BioDrugs. 2020;34:1–9. doi: 10.1007/s40259-019-00399-6. [DOI] [PubMed] [Google Scholar]
- 191.Mayo-Malasky P., Frishman W.H. Lipoprotein(a) testing and emerging therapies. Cardiol Rev. 2020;28:250–255. doi: 10.1097/CRD.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 192.Kim J., Eygeris Y., Gupta M., Sahay G. Self-assembled mRNA vaccines. Adv Drug Deliv Rev. 2021;170:83–112. doi: 10.1016/j.addr.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mauger D.M., Cabral B.J., Presnyak V., Su S.V., Reid D.W., Goodman B., et al. mRNA structure regulates protein expression through changes in functional half-life. Proc Natl Acad Sci U S A. 2019;116:24075–24083. doi: 10.1073/pnas.1908052116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Peeri M., Tuller T. High-resolution modeling of the selection on local mRNA folding strength in coding sequences across the tree of life. Genome Biol. 2020;21:63. doi: 10.1186/s13059-020-01971-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hussain M.M. Intestinal lipid absorption and lipoprotein formation. Curr Opin Lipidol. 2014;25:200–206. doi: 10.1097/MOL.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Packard C.J., Demant T., Stewart J.P., Bedford D., Caslake M.J., Schwertfeger G., et al. Apolipoprotein B metabolism and the distribution of VLDL and LDL subfractions. J Lipid Res. 2000;41:305–318. [PubMed] [Google Scholar]
- 197.Di L., Maiseyeu A. Low-density lipoprotein nanomedicines: mechanisms of targeting, biology, and theranostic potential. Drug Deliv. 2021;28:408–421. doi: 10.1080/10717544.2021.1886199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xu S.Q., Yang K.P., Li R., Zhang L. mRNA vaccine era-mechanisms, drug platform and clinical prospection. Int J Mol Sci. 2020;21:6582. doi: 10.3390/ijms21186582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rouf N.Z., Biswas S., Tarannum N., Oishee L.M., Muna M.M. Demystifying mRNA vaccines: an emerging platform at the forefront of cryptic diseases. RNA Biol. 2022;19:386–410. doi: 10.1080/15476286.2022.2055923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.He C.T., Qin M., Sun X. Highly pathogenic coronaviruses: thrusting vaccine development in the spotlight. Acta Pharm Sin B. 2020;10:1175–1191. doi: 10.1016/j.apsb.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Blakney A.K., McKay P.F., Yus B.I., Aldon Y., Shattock R.J. Inside out: optimization of lipid nanoparticle formulations for exterior complexation and in vivo delivery of saRNA. Gene Ther. 2019;26:363–372. doi: 10.1038/s41434-019-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ballesteros-Briones M.C., Silva-Pilipich N., Herrador-Canete G., Vanrell L., Smerdou C. A new generation of vaccines based on alphavirus self-amplifying RNA. Curr Opin Virol. 2020;44:145–153. doi: 10.1016/j.coviro.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Miao L., Zhang Y., Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hassett K.J., Higgins J., Woods A., Levy B., Xia Y., Hsiao C.J., et al. Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. J Control Release. 2021;335:237–246. doi: 10.1016/j.jconrel.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 205.Shi J., Huang M.W., Lu Z.D., Du X.J., Shen S., Xu C.F., et al. Delivery of mRNA for regulating functions of immune cells. J Control Release. 2022;345:494–511. doi: 10.1016/j.jconrel.2022.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hirahara K., Nakayama T. CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol. 2016;28:163–171. doi: 10.1093/intimm/dxw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cagigi A., Loré K. Immune responses induced by mRNA vaccination in mice, monkeys and humans. Vaccines (Basel) 2021;9:61. doi: 10.3390/vaccines9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pardi N., Hogan M.J., Naradikian M.S., Parkhouse K., Cain D.W., Jones L., et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;215:1571–1588. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Anderluzzi G., Lou G., Woods S., Schmidt S.T., Gallorini S., Brazzoli M., et al. The role of nanoparticle format and route of administration on self-amplifying mRNA vaccine potency. J Control Release. 2022;342:388–399. doi: 10.1016/j.jconrel.2021.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 211.Zhang M.M., Gao S., Yang D.J., Fang Y., Lin X.J., Jin X.C., et al. Influencing factors and strategies of enhancing nanoparticles into tumors. in vivo. Acta Pharm Sin B. 2021;11:2265–2285. doi: 10.1016/j.apsb.2021.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Oberli M.A., Reichmuth A.M., Dorkin J.R., Mitchell M.J., Fenton O.S., Jaklenec A., et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17:1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Phua K.K., Staats H.F., Leong K.W., Nair S.K. Intranasal mRNA nanoparticle vaccination induces prophylactic and therapeutic anti-tumor immunity. Sci Rep. 2014;4:5128. doi: 10.1038/srep05128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schlake T., Thess A., Fotin-Mleczek M., Kallen K.J. Developing mRNA-vaccine technologies. RNA Biol. 2012;9:1319–1330. doi: 10.4161/rna.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pascolo S. Synthetic messenger RNA-based vaccines: from scorn to hype. Viruses. 2021;13:270. doi: 10.3390/v13020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Iavarone C., O'hagan D.T., Yu D., Delahaye N.F., Ulmer J.B. Mechanism of action of mRNA-based vaccines. Expert Rev Vaccines. 2017;16:871–881. doi: 10.1080/14760584.2017.1355245. [DOI] [PubMed] [Google Scholar]
- 218.Verbeke R., Lentacker I., De Smedt S.C., Dewitte H. The dawn of mRNA vaccines: the COVID-19 case. J Control release. 2021;333:511–520. doi: 10.1016/j.jconrel.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Barbier A.J., Jiang A.Y., Zhang P., Wooster R., Anderson D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat Biotechnol. 2022;40:840–854. doi: 10.1038/s41587-022-01294-2. [DOI] [PubMed] [Google Scholar]
- 220.Plotkin S.A., Wang D., Oualim A., Diamond D.J., Kotton C.N., Mossman S., et al. The status of vaccine development against the human cytomegalovirus. J Infect Dis. 2020;221:S113–S122. doi: 10.1093/infdis/jiz447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.van Dülmen M., Rentmeister A. mRNA therapies: new hope in the fight against melanoma. Biochemistry. 2020;59:1650–1655. doi: 10.1021/acs.biochem.0c00181. [DOI] [PubMed] [Google Scholar]
- 222.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 223.Jabulowsky R., Loquai C., Mitzel-Rink H., Utikal J., Gebhardt C., Hassel J., et al. Abstract CT156: a first-in-human phase I/II clinical trial assessing novel mRNA-lipoplex nanoparticles encoding shared tumor antigens for immunotherapy of malignant melanoma. Cancer Res. 2018;78:CT156. [Google Scholar]
- 224.Curreri A., Sankholkar D., Mitragotri S., Zhao Z. RNA therapeutics in the clinic. Bioeng Transl Med. 2022 doi: 10.1002/btm2.10374. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Corbett K.S., Flynn B., Foulds K.E., Francica J.R., Boyoglu-Barnum S., Werner A.P., et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020;383:1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 227.Feng C., Li Y.J., Ferdows B.E., Patel D.N., Ouyang J., Tang Z.M., et al. Emerging vaccine nanotechnology: from defense against infection to sniping cancer. Acta Pharm Sin B. 2022;12:2206–2223. doi: 10.1016/j.apsb.2021.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chen Z.N., Du R.K., Galvan Achi J.M., Rong L.J., Cui Q.H. SARS-CoV-2 cell entry and targeted antiviral development. Acta Pharm Sin B. 2021;11:3879–3888. doi: 10.1016/j.apsb.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lu J., Lu G.L., Tan S.D., Xia J., Xiong H.L., Yu X.F., et al. A COVID-19 mRNA vaccine encoding SARS-CoV-2 virus-like particles induces a strong antiviral-like immune response in mice. Cell Res. 2020;30:936–939. doi: 10.1038/s41422-020-00392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hsieh C.L., Goldsmith J.A., Schaub J.M., DiVenere A.M., Kuo H.C., Javanmardi K., et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. 2020;369:1501–1505. doi: 10.1126/science.abd0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Schoenmaker L., Witzigmann D., Kulkarni J.A., Verbeke R., Kersten G., Jiskoot W., et al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm. 2021;601 doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Xiang R., Yu Z.S., Wang Y., Wang L.L., Huo S.S., Li Y.B., et al. Recent advances in developing small-molecule inhibitors against SARS-CoV-2. Acta Pharm Sin B. 2022;12:1591–1623. doi: 10.1016/j.apsb.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Paunovska K., Loughrey D., Dahlman J.E. Drug delivery systems for RNA therapeutics. Nat Rev Genet. 2022;23:265–280. doi: 10.1038/s41576-021-00439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Urits I., Swanson D., Swett M.C., Patel A., Berardino K., Amgalan A., et al. A review of patisiran (Onpattro®) for the treatment of polyneuropathy in people with hereditary transthyretin amyloidosis. Neurol Ther. 2020;9:301–315. doi: 10.1007/s40120-020-00208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Balwani M., Sardh E., Ventura P., Peiro P.A., Rees D.C., Stolzel U., et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N Engl J Med. 2020;382:2289–2301. doi: 10.1056/NEJMoa1913147. [DOI] [PubMed] [Google Scholar]
- 238.Garrelfs S.F., Frishberg Y., Hulton S.A., Koren M.J., O'Riordan W.D., Cochat P., et al. Lumasiran, an RNAi therapeutic for primary hyperoxaluria type 1. N Engl J Med. 2021;384:1216–1226. doi: 10.1056/NEJMoa2021712. [DOI] [PubMed] [Google Scholar]
- 239.Ray K.K., Wright R.S., Kallend D., Koenig W., Leiter L.A., Raal F.J., et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382:1507–1519. doi: 10.1056/NEJMoa1912387. [DOI] [PubMed] [Google Scholar]
- 240.Tatiparti K., Sau S., Kashaw S.K., Iyer A.K. siRNA delivery strategies: a comprehensive review of recent developments. Nanomaterials. 2017;7:77. doi: 10.3390/nano7040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tang G. siRNA and miRNA: an insight into RISCs. Trends Biochem Sci. 2005;30:106–114. doi: 10.1016/j.tibs.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 242.Setten R.L., Rossi J.J., Han S.P. The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov. 2019;18:421–446. doi: 10.1038/s41573-019-0017-4. [DOI] [PubMed] [Google Scholar]
- 243.Kanasty R., Dorkin J.R., Vegas A., Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 244.Weng Y.H., Li C.H., Yang T., Hu B., Zhang M.J., Guo S., et al. The challenge and prospect of mRNA therapeutics landscape. Biotechnol Adv. 2020;40 doi: 10.1016/j.biotechadv.2020.107534. [DOI] [PubMed] [Google Scholar]
- 245.Rejman J., Tavernier G., Bavarsad N., Demeester J., De Smedt Scjjocr mRNA transfection of cervical carcinoma and mesenchymal stem cells mediated by cationic carriers. J Control release. 2010;147:385–391. doi: 10.1016/j.jconrel.2010.07.124. [DOI] [PubMed] [Google Scholar]
- 246.Pollock K.M., Cheeseman H.M., Szubert A.J., Libri V., Boffito M., Owen D., et al. Safety and immunogenicity of a self-amplifying RNA vaccine against COVID-19: COVAC1, a phase I, dose-ranging trial. EClinicalMedicine. 2022;44 doi: 10.1016/j.eclinm.2021.101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Younis M.A., Tawfeek H.M., Abdellatif A.A.H., Abdel-Aleem J.A., Harashima H. Clinical translation of nanomedicines: challenges, opportunities, and keys. Adv Drug Deliv Rev. 2022;181 doi: 10.1016/j.addr.2021.114083. [DOI] [PubMed] [Google Scholar]
- 248.Xiao Q.Q., Zoulikha M., Qiu M., Teng C., Lin C.S., Li X.T., et al. The effects of protein corona on in vivo fate of nanocarriers. Adv Drug Deliv Rev. 2022;186 doi: 10.1016/j.addr.2022.114356. [DOI] [PubMed] [Google Scholar]
- 249.Ryan G.M., Kaminskas L.M., Porter C.J. Nano-chemotherapeutics: maximising lymphatic drug exposure to improve the treatment of lymph-metastatic cancers. J Control Release. 2014;193:241–256. doi: 10.1016/j.jconrel.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 250.Irvine D.J., Swartz M.A., Szeto G.L. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jiang H., Wang Q., Sun X. Lymph node targeting strategies to improve vaccination efficacy. J Control Release. 2017;267:47–56. doi: 10.1016/j.jconrel.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 252.Xiao Q.Q., Li X.T., Li Y., Wu Z.F., Xu C.J., Chen Z.J., et al. Biological drug and drug delivery-mediated immunotherapy. Acta Pharm Sin B. 2021;11:941–960. doi: 10.1016/j.apsb.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dilliard S.A., Cheng Q., Siegwart D.J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2109256118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Maier M.A., Jayaraman M., Matsuda S., Liu J., Barros S., Querbes W., et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol Ther. 2013;21:1570–1578. doi: 10.1038/mt.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Latroche C., Gitiaux C., Chrétien F., Desguerre I., Mounier R., Chazaud B. Skeletal muscle microvasculature: a highly dynamic lifeline. Physiology. 2015;30:417–427. doi: 10.1152/physiol.00026.2015. [DOI] [PubMed] [Google Scholar]
- 256.Zhu D.W., Hu C.Y., Fan F., Qin Y., Huang C.L., Zhang Z.M., et al. Co-delivery of antigen and dual agonists by programmed mannose-targeted cationic lipid-hybrid polymersomes for enhanced vaccination. Biomaterials. 2019;206:25–40. doi: 10.1016/j.biomaterials.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 257.Song T.T., Xia Y.F., Du Y.Q., Chen M.W., Qing H., Ma G. Engineering the deformability of albumin-stabilized emulsions for lymph-node vaccine delivery. Adv Mater. 2021;33 doi: 10.1002/adma.202100106. [DOI] [PubMed] [Google Scholar]
- 258.Perche F., Benvegnu T., Berchel M., Lebegue L., Pichon C., Jaffrès P.A., et al. Enhancement of dendritic cells transfection in vivo and of vaccination against B16F10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA. Nanomedicine. 2011;7:445–453. doi: 10.1016/j.nano.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 259.Szebeni J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol Immunol. 2014;61:163–173. doi: 10.1016/j.molimm.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 260.Han X.X., Zhang H.W., Butowska K., Swingle K.L., Alameh M.G., Weissman D., et al. An ionizable lipid toolbox for RNA delivery. Nat Commun. 2021;12:7233. doi: 10.1038/s41467-021-27493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gillmore J.D., Gane E., Taubel J., Kao J., Fontana M., Maitland M.L., et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med. 2021;385:493–502. doi: 10.1056/NEJMoa2107454. [DOI] [PubMed] [Google Scholar]
- 262.Chen S., Zaifman J., Kulkarni J.A., Zhigaltsev I.V., Tam Y.K., Ciufolini M.A., et al. Dexamethasone prodrugs as potent suppressors of the immunostimulatory effects of lipid nanoparticle formulations of nucleic acids. J Control Release. 2018;286:46–54. doi: 10.1016/j.jconrel.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 263.Ma F.H., Yang L., Sun Z.R., Chen J.J., Rui X.H., Glass Z., et al. Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Sci Adv. 2020;6 doi: 10.1126/sciadv.abb4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.