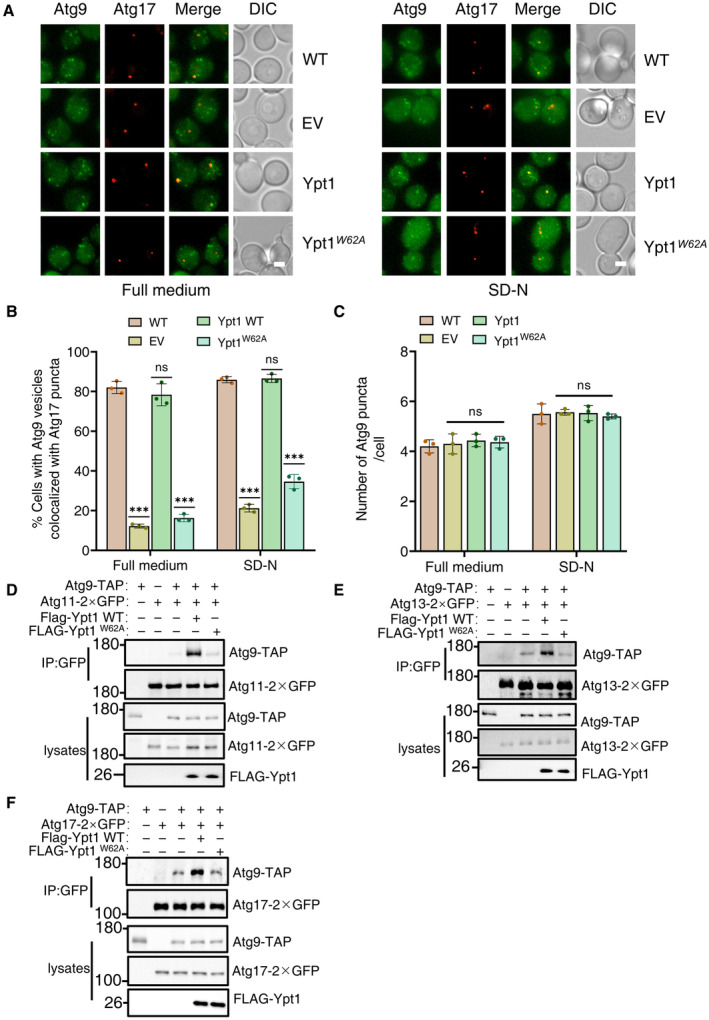
Figure 4. Ypt1‐Atg23 binding is required for the PAS localization of Atg9 vesicles.
-
AWild‐type yeast strains co‐expressing Atg17‐2×mCherry and Atg9‐2×GFP or AID‐3×HA‐Ypt1 yeast strains co‐expressing Atg17‐2×mCherry and Atg9‐2×GFP transformed with empty vector, FLAG‐Ypt1, or FLAG‐Ypt1W62A plasmids were treated with IAA for 2 h to degrade endogenous AID‐3×HA‐Ypt1, and then subjected to SD‐N for 0 or 1 h. Images of cells were obtained using fluorescence microscopy. Scale bar, 2 μm.
-
BCells from (A) were quantified for the number of cells in which Atg9‐2 × GFP colocalized with Atg17‐2 × mCherry puncta. n = 300 cells were pooled from three independent experiments. Data are shown as mean ± SD. ***P < 0.001; ns, no significance; two‐tailed Student's t‐tests were used.
-
CCells from (A) were quantified for the number of Atg9‐2 × GFP. n = 300 cells were pooled from three independent experiments. Data are shown as mean ± SD. ns, no significance; two‐tailed Student's t‐tests were used.
-
D–FAID‐3×HA‐Ypt1 cells co‐expressing Atg9‐TAP and Atg11‐2×GFP, Atg13‐2×GFP, or Atg17‐2×GFP transformed with empty vector, FLAG‐Ypt1, or FLAG‐Ypt1 W62A plasmids were grown to log phase, and then subjected to SD‐N for 1 h. Cell lysates were immunoprecipitated with anti‐GFP agarose beads and then analyzed by western blot using the indicated antibody.
Source data are available online for this figure.
