Figure 1. Flow of Participants in the SURPASS-6 Trial.
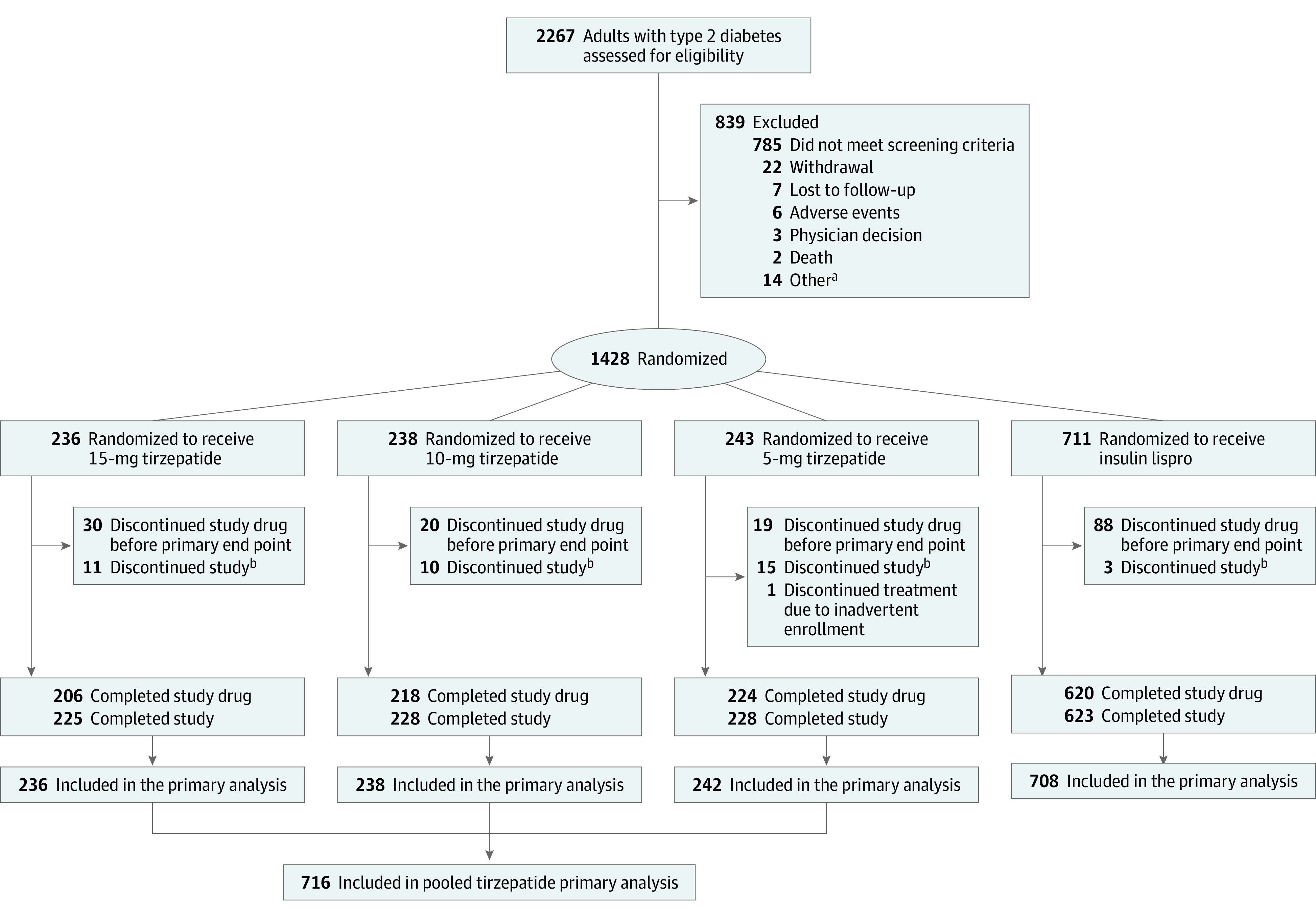
See Table 3 for the details of the adverse events that led to study treatment discontinuation.
aIncludes 1 participant each who did not meet baseline age criteria, had chronic or acute pancreatitis or hepatitis, receiving drugs that directly affect gastrointestinal motility, or have a known clinically significant gastric emptying abnormality, have history of history of diabetic ketoacidosis or hyperosmolar state/coma, have family or personal history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2, have evidence of an autoimmune abnormality, have a history of any other condition that may preclude the participant from following and completing the protocol, are pregnant or breastfeeding, have participated in a clinical study involving an investigational product, or have previously completed/discontinued from this study or any other study investigating tirzepatide.
bStudy discontinuations before, after, or at the primary end point visit were included.
