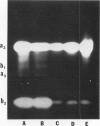
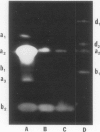
Abstract
Most of the activity of an α-amylase present in crude pea (Pisum sativum L. cv Laxton's Progress No. 9) leaf preparations cannot be found in isolated pea leaf protoplasts. The same extrachloroplastic α-amylase is present in pea stems, representing approximately 6% of total stem amylolytic activity and virtually all of the α-amylase activity. By a simple infiltration-extraction procedure, the majority (87%) of this α-amylase activity was recovered from the pea stem apoplast without significantly disrupting the symplastic component of the tissue. Only 3% of the β-amylase activity and less than 2% of other cellular marker enzymes were removed during infiltration-extraction.
Full text
PDF
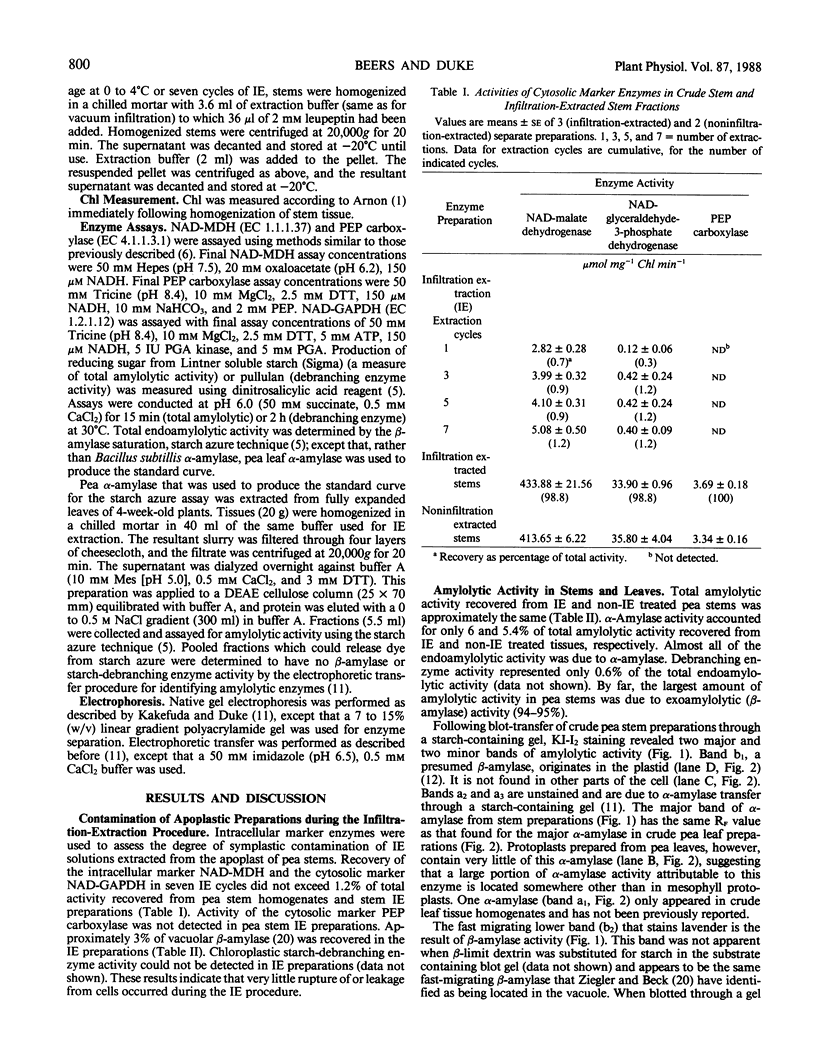
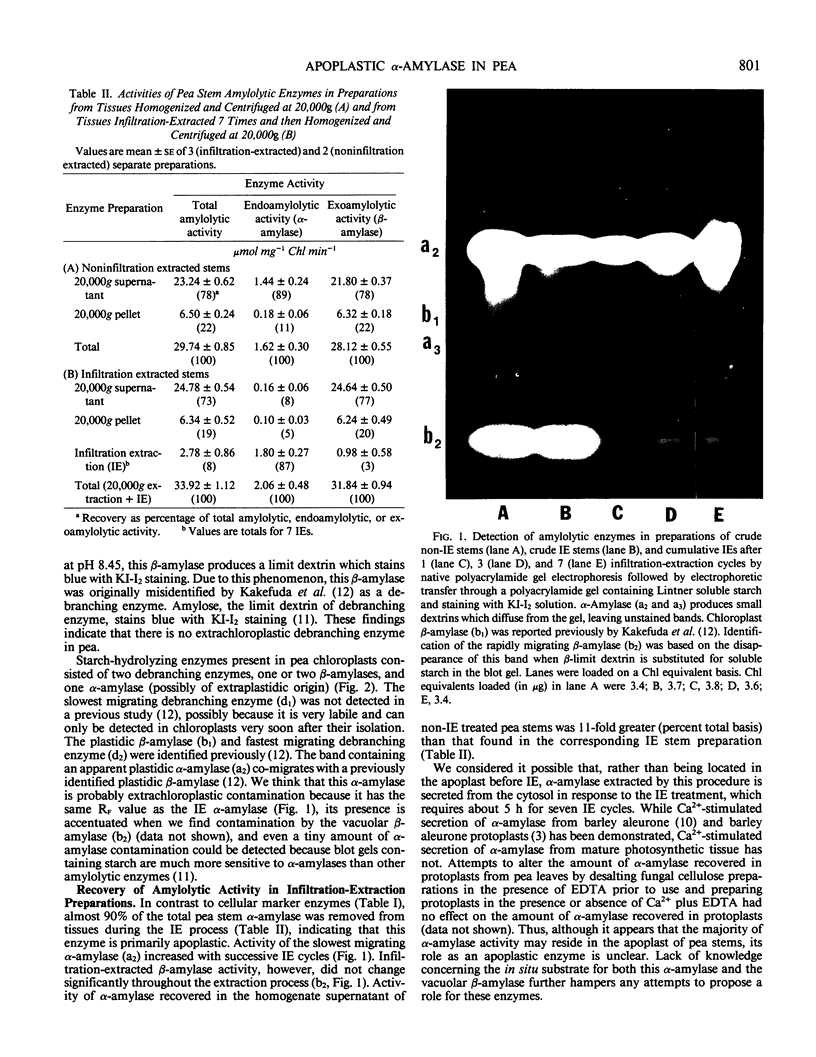

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. S., Cornejo M. J., Huang C. N., Jones R. L. Ca-stimulated secretion of alpha-amylase during development in barley aleurone protoplasts. Plant Physiol. 1986 Oct;82(2):566–574. doi: 10.1104/pp.82.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlert D. C., Duke S. H. Specific Determination of alpha-Amylase Activity in Crude Plant Extracts Containing beta-Amylase. Plant Physiol. 1983 Feb;71(2):229–234. doi: 10.1104/pp.71.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. H., Schrader L. E., Henson C. A., Servaites J. C., Vogelzang R. D., Pendleton J. W. Low root temperature effects on soybean nitrogen metabolism and photosynthesis. Plant Physiol. 1979 May;63(5):956–962. doi: 10.1104/pp.63.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehner M., Conn E. E. The Linamarin beta-Glucosidase in Costa Rican Wild Lima Beans (Phaseolus lunatus L.) Is Apoplastic. Plant Physiol. 1987 Aug;84(4):1296–1300. doi: 10.1104/pp.84.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Hanson A. D., Chandler P. C. Water stress enhances expression of an alpha-amylase gene in barley leaves. Plant Physiol. 1986 Feb;80(2):350–359. doi: 10.1104/pp.80.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakefuda G., Duke S. H. Electrophoretic transfer as a technique for the detection and identification of plant amylolytic enzymes in polyacrylamide gels. Plant Physiol. 1984 May;75(1):278–280. doi: 10.1104/pp.75.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi C., Preiss J. Amylopectin degradation in pea chloroplast extracts. Plant Physiol. 1978 Feb;61(2):218–220. doi: 10.1104/pp.61.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Greenberg E., Kuhn D. N., Preiss J. Subcellular localization of the starch degradative and biosynthetic enzymes of spinach leaves. Plant Physiol. 1979 Aug;64(2):187–192. doi: 10.1104/pp.64.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Preiss J. Starch Degradation in Spinach Leaves: ISOLATION AND CHARACTERIZATION OF THE AMYLASES AND R-ENZYME OF SPINACH LEAVES. Plant Physiol. 1980 Nov;66(5):870–876. doi: 10.1104/pp.66.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Bulpin P. V., ap Rees T. Pathway of starch breakdown in photosynthetic tissues of Pisum sativum. Biochim Biophys Acta. 1978 Nov 15;544(1):200–214. doi: 10.1016/0304-4165(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Ziegler P., Beck E. Exoamylase activity in vacuoles isolated from pea and wheat leaf protoplasts. Plant Physiol. 1986 Dec;82(4):1119–1121. doi: 10.1104/pp.82.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]