Abstract
In recent years, it has been shown that a nonclassical, major histocompatibility complex-independent system (i.e., CD1-restricted T-cell responses) is involved in T-cell immunity against nonpeptide antigens. The CD1 system appears to function by presenting microbial lipid antigens to specific T cells, and the antigens so far identified include several known constituents of mycobacterial cell walls. Among the four known human CD1 isoforms, the CD1b protein is the best characterized with regard to its antigen-presenting function. Expression of CD1b is upregulated on human blood monocytes upon exposure to granulocyte/macrophage-colony stimulating factor, alone or in combination with interleukin-4 (IL-4) (S. A. Porcelli, Adv. Immunol. 59:1–98, 1995). Rifampin (RFP) and its derivatives are widely used for chemoprophylaxis or chemotherapy against Mycobacterium tuberculosis. However, this agent was found to reduce the mitogen responsiveness of human B and T lymphocytes, chemotaxis, and delayed-type hypersensitivity. The present study extends the immunopharmacological profile of RFP by examining its effects on CD1b expression by human peripheral blood monocytes exposed to GM-CSF plus IL-4. The results showed that clinically attainable concentrations (i.e., 2 or 10 μg/ml for 24 h) of the agent produced a marked increase in CD1b expression on the plasma membrane, as evaluated by fluorescence-activated cell sorter analysis, whereas it had no effect on cytosolic fractions, as indicated by Western blot analysis. This was found to be the result of increased CD1b gene expression, as shown by Northern blot analysis of CD1b mRNA. These results suggest that RFP could be of potential value in augmenting the CD1b-restricted antigen recognition system, thereby enhancing protective cellular immunity to M. tuberculosis.
The incidence of mycobacterial infections has rapidly increased in recent years. One of the principal causes of this phenomenon appears to be the high susceptibility of human immunodeficiency virus-positive persons to mycobacterial pathogens (3, 11, 17).
A large amount of experimental and clinical evidence showing that T-cell-mediated immune responses play a significant role in resistance against mycobacteria is now available (18, 28). Subpopulations of T cells that are involved in antimycobacterial immunity include CD3+ lymphocytes bearing the αβ T-cell receptor (TCR), predominantly of the CD4+ phenotype (18), and γδ TCR T lymphocytes (28). Effector CD4+ T cells are sensitized with mycobacterium-derived peptides presented by antigen-presenting cells in association with the class II major histocompatibility complex (MHC, 25). Immune lymphocytes show a Th1-like response pattern, being cytotoxic for mycobacterial targets and capable of secreting gamma interferon upon challenge with the relevant antigen (18, 28).
In recent years, growing interest has been elicited by a nonclassical, MHC-independent system that appears to be additionally involved in T-cell responses against mycobacteria. In this case, the human antigen-presenting molecule is the group I, nonpolymorphic CD1b protein (1, 2, 15, 19) expressed by cytokine-activated macrophages (20). The antigens presented by the CD1b molecule belong to a variety of nonpeptide macromolecules (20). Among them, mycolic acids, lipoarabinomannan, and other lipid structures associated with the mycobacterial cell wall are believed to be involved in CD1-dependent host resistance against tuberculosis. Lipoarabinomannan is taken up by a macrophage mannose receptor that carries the antigen to macrophage endosomes, where it is loaded onto CD1b molecules (21).
In this system, many of the responder cells come from the CD4− 8− phenotypic subset of CD3+, αβ TCR T cells. These cells, referred to as double-negative αβ T lymphocytes (20), proliferate and generate cytotoxic clones following interaction with mycobacterial glycolipids presented by CD1b+ monocytes preactivated with granulocyte/macrophage-colony stimulating factor (GM-CSF), alone or in combination with interleukin-4 (IL-4) (20). More recently, CD8+, αβ TCR T-cell clones with similar properties have also been demonstrated (26).
Rifampin (RFP) and a number of its derivatives (e.g., rifabutin) are widely used for therapy against Mycobacterium tuberculosis in immunocompromised patients (16) or to treat various types of mycobacterial infections provoked by atypical strains (i.e., M. avium; 4). However, previous studies showed that RFP reduces humoral and cell-mediated immunity (9, 10, 14, 29). These observations suggested the possibility that antitubercular chemotherapy with RFP would attenuate the functional activity of the immune system, with possible negative effects on resistance against mycobacterial infections. To study the possible effects of this antibiotic on macrophage function relative to antigen presentation by CD1b molecules, CD1b molecule expression has been studied in vitro in human peripheral blood monocytes exposed to GM-CSF plus IL-4, alone or in the presence of RFP. The results showed that clinically attainable concentrations of the agent increased CD1b expression, thus suggesting that the antibiotic could be of potential value in improving the CD1b-restricted antigen recognition system.
MATERIALS AND METHODS
Reagents and antibodies.
Recombinant human GM-CSF and IL-4 were obtained from Sandoz (Milan, Italy) and Genzyme (Cambridge, Mass.), respectively. Phenylmethylsulfonyl fluoride, EGTA, Triton X-100, leupeptin, aprotinin, soybean trypsin inhibitor, and RFP were obtained from Sigma Chemical Co. (St. Louis, Mo.).
A purified, fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody (MAb) recognizing CD1b (SN13, immunoglobulin G1k [IgG1k], K5-1B8 clone) and an FITC-conjugated MAb recognizing CD14 (IgG2a, UCHM1 clone) were obtained from Ancell (Bayport, Minn.). FITC-conjugated mouse IgG1 or IgG2a was used as a negative control (Becton Dickinson, Oxnard, Calif.). Rabbit polyclonal antiserum recognizing the denatured CD1b protein for Western blot analysis was obtained in our laboratory as previously described (8).
Preparation of human AMNC.
Peripheral blood mononuclear cells were separated from heparinized whole blood, obtained from healthy donors, on a Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) gradient, washed twice in RPMI 1640 medium (HyClone Europe Ltd., Cramlington, United Kingdom), and resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum (HyClone), 10 mM HEPES (Flow Laboratories, McLean, Va.), 2 mM l-glutamine (Flow), 0.8 mM nonessential amino acids (GIBCO, Paisley, Scotland), 0.4 mM essential amino acids, and 50 μM 2-mercaptoethanol (Sigma) (hereafter referred to as complete medium). Adherent mononuclear cells (AMNC) were removed from samples by plastic adherence at 37°C for 2 h, followed by extensive washing with warm RPMI 1640.
Immunofluorescence staining and cytofluorimetric analysis.
Cultured cells were washed twice in phosphate-buffered saline supplemented with 0.1% bovine serum albumin and 0.02% sodium azide (PBS-A; Sigma). Cells (106) were then suspended in 50 μl of PBS-A containing the appropriate MAb. For a negative control, cells were incubated with FITC-conjugated IgG1 or IgG2a. Samples were incubated at 4°C for 30 min and then washed twice in PBS-A. The labeled samples were analyzed with a FACScan. Data on 104 viable cells were collected as forward and side-angle light scatter. Data analysis was performed by using Lysis II software (Becton Dickinson).
Preparation of cell extracts.
Cells were washed extensively with phosphate-buffered saline. The cell pellet was suspended in 5 volumes of lysis buffer (25 mM HEPES [pH 7.5], 2.5 mM MgCl2, 2.5 mM EGTA, 50 mM 2-mercaptoethanol, 200-μg/ml leupeptin, 5-μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 400-μg/ml soybean trypsin inhibitor), sonicated at 4°C for 5 s, and centrifuged at 100,000 × g and 4°C for 1 h. The supernatant was collected and designated as the cytosol fraction. The pellet was resuspended in lysis buffer containing 1% Triton X-100, sonicated for 5 s, and centrifuged at 15,000 × g and 4°C in a microcentrifuge for 10 min. The supernatant was collected and defined as the membrane fraction.
Immunoblotting.
Membrane and cytosol fractions were separated in sodium dodecyl sulfate (SDS)–10% (wt/vol) polyacrylamide gels as described by Laemmli (13) and transferred to nitrocellulose filters as described by Towbin et al. (27), with a Bio-Rad electrophoretic miniblotting apparatus (Bio-Rad, Hercules, Calif.). Transfer was carried out at 25 V and 4°C for 14 h. After transfer, membranes were incubated with 3% (wt/vol) nonfat dry milk (Bio-Rad) in TBS (20 mM Tris-HCl [pH 7.5], 0.9% NaCl) with gentle agitation for 1 h. The membranes were then incubated at room temperature with rabbit anti-CD1b serum diluted 1:2,000 in TBS containing 0.05% Tween 20 (TBST) for 30 min. Thereafter, the membranes were washed twice with TBST and incubated with an alkaline phosphatase-coupled secondary antibody diluted 1:7,500 in TBST for 1 h. The bands were visualized by using the Protoblot (Promega Biotec, Madison, Wis.) reagents in accordance with the procedures provided by the manufacturer.
Northern blot analysis.
Total RNA was extracted by the guanidinium thiocyanate method described by Chomczynski and Sacchi (5). Fifteen micrograms of total RNA was denatured in 2.2 M formaldehyde–50% formamide at 65°C and fractionated in a 1.2% agarose gel containing 2.2 M formaldehyde. RNA was then transferred to a GeneScreen Plus nylon membrane (Dupont, NEN Research products, Boston, Mass.) in 10× SSC (1× SSC is 0.1 M NaCl plus 0.015 M sodium citrate). Prehybridization and hybridization were performed in accordance with the manufacturer’s instructions. Briefly, filters were prehybridized at 42°C in 50% formamide–10% dextran sulfate–1 M NaCl–1% SDS for 2 h. Hybridization was then performed at the same temperature in the prehybridization solution following addition of denatured salmon sperm DNA (100 μg/ml) and of the probe labeled with [α-32P]dCTP (3,000 Ci/mmol; Dupont), using a random primed labeling kit (Boehringer Mannheim, Indianapolis, Ind.). Filters were washed with 2× SSC at room temperature for 5 min, with 2× SSC containing 1% SDS at 60°C for 30 min, and then with 0.1× SSC at room temperature for 30 min. Autoradiography was performed at −80°C with XAR-5 film (Kodak, Rochester, N.Y.).
Detection of the CD1b-specific transcript was done with a 266-bp cDNA probe corresponding to the second exon of CD1b, which encodes the extracellular domain of mature CD1b, designated α1 (15). This probe was obtained by PCR amplification of 1 μg of genomic DNA extracted from human monocytes by standard procedures (24). The PCR was performed by adding a DNA template to a solution (total volume, 100 μl) containing 1× PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 1.5 mM MgCl2, 0.01% gelatin) and 200 μM (each) dCTP, dATP, dGTP, and dTTP. Twenty picomoles each of two synthetic oligonucleotides with the sequences 5′-CCTTCCAGGGGCCGACCTCCTTT-3′ and 5′-TTCATCTGGAAATCACCGGCA-3′ were added to the mixture. Taq DNA polymerase (2 U; Boehringer Mannheim) was added to the PCR mixture, and DNA amplification was performed for 30 cycles in a DNA thermal cycler (Perkin Elmer Cetus, Norwalk, Conn.). Each cycle consisted of denaturation at 95°C for 1 min, annealing at 58°C for 1 min, and extension at 72°C for 2 min.
A glyceraldehyde phosphate dehydrogenase (GAPDH) probe, corresponding to a 0.9-kb EcoRI fragment of the GAPDH gene, was used as a control for Northern blot analysis. This probe was kindly provided by R. Dalla Favera (Department of Pathology, Columbia University, New York, N.Y.).
RESULTS
Effect of RFP on CD1b expression at the AMNC membrane level.
Freshly prepared AMNC were tested for CD14 expression to evaluate the amount of monocytic cells present at the beginning of each experiment. In most cases, the percentage of CD14+ cells ranged from 70 to 85%.
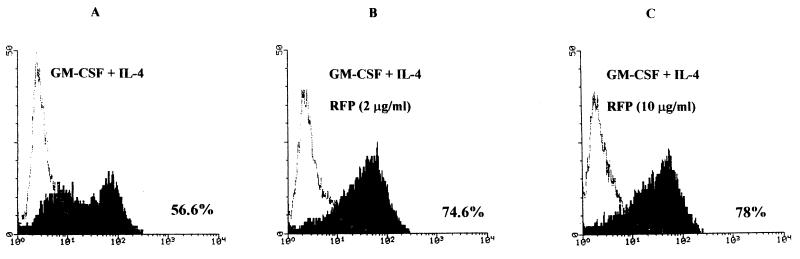
Washed AMNC, seeded in 25-cm2 tissue culture flasks (50 × 106 cells/flask), were incubated with GM-CSF (10 IU/ml) alone, IL-4 (200 IU/ml) alone, or GM-CSF and IL-4 together. Additional groups, untreated or treated with the cytokines, were exposed to RFP at 2 to 10 μg/ml. On day 3 of culture, the total number of viable AMNC (i.e., cells excluding trypan blue) in each group was determined. Thereafter, the cells were washed, counted again, and tested for CD1b expression by fluorescence-activated cell sorter analysis. The results indicate that treatment with RFP (2 or 10 μg/ml) was not toxic for AMNC, since no difference in cell number or viability was detected between groups not treated with the antibiotic and the corresponding groups subjected to RFP treatment (data not shown). The results of fluorescence-activated cell sorter analysis performed with a MAb recognizing the native form of the CD1b molecule, illustrated in Fig. 1 and described in the legend, show that (i) marginal levels of the CD1b molecule were present on the membranes of untreated AMNC, whereas treatment with IL-4 alone resulted in a slight increase of the percentage of CD1b-positive cells; (ii) marked expression of the CD1b epitope was detectable on the membranes of AMNC treated with GM-CSF alone or in combination with IL-4; (iii) the percentages of cells expressing CD1b molecules in AMNC exposed to GM-CSF plus IL-4 and RFP (2 or 10 μg/ml) were higher than those of cells treated with the combination of cytokines without the antibiotic (Similarly, treatment with RFP increased by 20% the percentage of CD1b-positive cells in the group exposed to GM-CSF alone.); and (iv) exposure of AMNC to RFP (2 or 10 μg/ml) did not modify the low percentages of CD1b-positive cells in the groups not treated with the cytokines or treated with IL-4 alone.
FIG. 1.
Flow cytometry analysis of CD1b expression in AMNC pretreated with GM-CSF (10 IU/ml) plus IL-4 (200 IU/ml) alone (A) or with RFP at 2 (B) or 10 (C) μg/ml. Analysis was performed after 3 days of culture. Isotype-matched control MAb results are shown as a faint profile, and CD1b results are shown as a solid profile. Percent values relative to cells positive for CD1b molecules (range of four separate experiments; data not shown) were as follows: untreated AMNC, 1 to 3%; AMNC treated with IL-4 alone, 13 to 15%; AMNC treated with GM-CSF alone, 50 to 53%; AMNC treated with GM-CSF plus RFP (2 or 10 μg/ml), 60 to 70%. Exposure of AMNC to RFP (2 or 10 μg/ml) did not modify the limited percentage of CD1b-positive cells in the untreated or IL-4-treated groups.
Influence of RFP on induction of the CD1b protein in cytosolic and membrane fractions of AMNC.
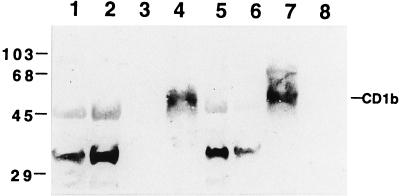
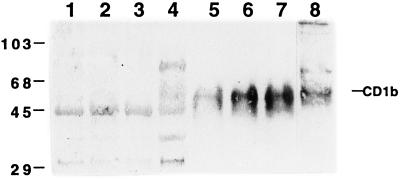
Western blot analysis of cytosol and cell membrane preparations obtained from nonstimulated or stimulated AMNC, with or without RFP treatment, was performed by using the rabbit polyclonal antiserum obtained in our laboratory. It should be noted that the commercially available antibody against CD1b was unable to react with the CD1b protein in a Western blot analysis (data not shown). The polyclonal antibody used in the present study recognizes the denatured form of the CD1b protein and is suitable for Western blot assays. The specificity of the antibody was preliminarily examined by immunoblotting with a human B-lymphoblastoid cell line (C1R) permanently transfected with the expression vector pSRα-Neo containing the CD1b cDNA (C1R/CD1b cells). Control C1R cells were obtained following transfection with a mock plasmid (C1R/MOCK cells). A polyclonal antibody revealed a specific band of about 50 kDa only in the membrane fraction of C1R/CD1b cells and AMNC activated with GM-CSF plus IL-4 (Fig. 2). Control or stimulated AMNC contained a cytosolic immunoreactive 45-kDa protein which was also present in C1R/MOCK cells. Figure 3 shows that RFP markedly increased the level of membrane CD1b in cytokine-activated AMNC but had no effect on cytosolic fractions.
FIG. 2.
Specificity of the polyclonal CD1b antibody. Shown is an immunoblot of membrane (lanes 3, 4, 7, and 8) and cytosol (lanes 1, 2, 5, and 6) CD1b expressed in AMNC (lanes 1 to 4) and in human B-lymphoblastoid cell line C1R after transfection with the expression vector pSRα-Neo containing CD1b cDNA (lanes 5 and 7) or with a mock vector (lanes 6 and 8). Lanes: 1 and 3, unstimulated AMNC; 2 and 4 AMNC treated with GM-CSF plus IL-4. The values on the left represent molecular size standards in kilodaltons.
FIG. 3.
Effect of RFP treatment on CD1b in membrane and cytosol fractions. Cells were treated with GM-CSF plus IL-4 (lanes 1 to 3 and 5 to 7) in the presence of RFP at 2 (lanes 2 and 6) or 10 (lanes 3 and 7) μg/ml. Cell homogenates were separated into membrane and cytosol fractions. Each fraction (20 μg) was separated by SDS-polyacrylamide gel electrophoresis and visualized by immunoblotting with the polyclonal antibody against CD1b. Lanes 1 to 4, cytosol fractions from AMNC (lanes 1 to 3) and C1R/CD1b cells (lane 4); 5 to 8, membrane fractions from AMNC (lanes 5 to 7) and C1R/CD1b cells (lane 8). The values on the left are molecular size standards (in kilodaltons).
Influence of RFP on CD1b mRNA levels.
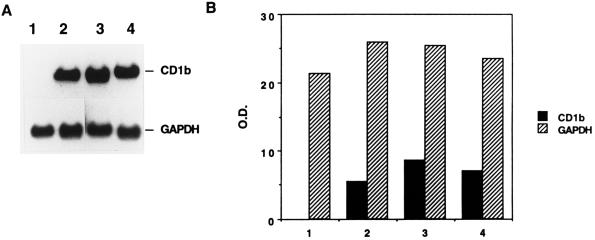
Northern blot analysis of total RNA hybridized with a CD1b cDNA probe showed the presence of a transcript of approximately 2.0 kb in AMNC stimulated with GM-CSF plus IL-4 (Fig. 4A, lane 2). Additional treatment of AMNC with RFP (10 [lane 3] or 2 [lane 4] μg/ml) increased the level of CD1b mRNA as evidenced by the results of a representative experiment illustrated in Fig. 4A (lanes 3 and 4 versus lane 2) and by the densitometric analysis performed on bands obtained in the Northern blot analysis (Fig. 4B). Hybridization of a similar blot containing the same samples with a GAPDH probe resulted in comparable hybridization signals in these lanes (Fig. 4). In addition, exposure of AMNC to a high concentration of RFP (i.e., 50 μg/ml) along with GM-CSF and IL-4, resulted in a marked (i.e., more-than-threefold) increase in the amount of the specific CD1b transcript (data not shown). The integrity of RNA samples was confirmed by ethidium bromide staining of the gel before blotting (data not shown).
FIG. 4.
Analysis of CD1b transcripts in cytokine-activated AMNC exposed to RFP. (A) Northern blot analysis of a human CD1b transcript. Lanes: 1, unstimulated AMNC; 2, GM-CSF–IL-4-treated AMNC; 3, GM-CSF–IL-4-treated AMNC in the presence of RFP at 10 μg/ml; 4, GM-CSF–IL-4-treated AMNC in the presence of RFP at 2 μg/ml. The results shown are from a representative experiment of three with comparable results. (B) Results of blot scanning by densitometer. Optical densities (O.D.) are expressed in arbitrary units.
DISCUSSION
The results of the present study indicate that RFP, at concentrations of clinical relevance, increases CD1b expression of cytokine-activated AMNC, as assessed by cytofluorimetry analysis. This observation was further confirmed by Western and Northern blot analyses.
No data are available that explain the mechanisms underlying this phenomenon. The observation that an RFP-mediated increase in CD1b expression occurs in AMNC activated by GM-CSF or by GM-CSF plus IL-4 without evidence of drug-induced cytotoxicity seems to rule out any selection mechanism favoring the survival of CD1b-positive cells. It is known that the antibiotic impairs the functional RNA polymerase activity of bacteria by blocking transfer of the nascent RNA to the RNA-binding site of the β unit of the enzyme (30). Therefore, it is reasonable to suggest the hypothesis that RFP would impair the transcription of a DNA binding factor acting as a negative regulator of CD1b gene transcription in AMNC during cytokine-mediated induction of the antigen-presenting molecule.
The Northern blot analysis data show that the CD1b gene transcript is detectable only in AMNC following treatment with GM-CSF (data not shown) or GM-CSF plus IL-4 (Fig. 4). Additional treatment with RFP increased the level of CD1b mRNA. Cytokine-induced appearance of the CD1b protein on AMNC is due mainly to induction of CD1b gene transcription, rather than to protein translocation from the cytoplasm to the cell membrane. In this context, it should be noted that the RFP-mediated increase in CD1b in cytokine-activated AMNC is also not the result of augmented translocation of the antigen to the plasma membrane but the consequence of an actual increase in CD1b protein synthesis. This is further supported by Western blot analysis showing that the CD1b protein is almost undetectable in the cytoplasm of nonactivated AMNC or of cytokine-activated AMNC not treated or exposed to RFP (Fig. 3).
It remains to be established whether the RFP-mediated increase in CD1b protein is the result of an actual increase in gene transcription or of augmented mRNA stability.
Reports from the literature on the immunotoxicological profile of RFP suggest that this drug possesses modest but significant depressive effects on immune responses (9, 10, 14, 22, 29). One of the molecular mechanisms underlying the presumed immunotoxic effects of RFP appears to be related to inhibition of protein synthesis in immunocompetent cells induced by the antitubercular agent (12). In addition, mycobacteria are known to downregulate helper T-cell activity and B7 expression in infected macrophages, thus reducing costimulatory signals required for T-cell responses to mycobacterial peptides (23). It must be pointed out that the efficacy of RFP and its analogs is conditioned by efficient immune responses against the infectious agent (6, 7). Therefore, immunodepression induced by mycobacteria and by the antibiotic itself should limit the therapeutic effectiveness of RFP. However, a number of studies seem to confirm that non-MHC-restricted CD1b molecules would play a pivotal role in host resistance against M. tuberculosis. The results of the present investigation suggest that RFP could amplify cytokine-mediated induction of the CD1b molecule on monocyte membranes, thus contributing positively to mycobacterial antigen presentation and, presumably, to T-cell responses against the infectious agent.
In conclusion, the present report illustrates, for the first time, a possible role that could be played by RFP in the CD1-dependent system. Actually, a rather complex immunopharmacological profile of the antibiotic appears to originate from the available data concerning its interaction with the immune system. The modest depression of classical MHC-dependent, cell-mediated T-cell immunity induced by RFP could be balanced by its favorable effects on lipid and glycolipid antigen presentation mediated by CD1b molecules on peripheral blood monocytes. It should be added that RFP does not show any detrimental effect on the functional activity of double-negative T cells (data not shown), which are involved in CD1b-restricted glycolipid antigen recognition and killing of target cells carrying the relevant antigen. In any case, since no data are available to establish the relative importance of classical MHC-restricted and nonclassical CD1-restricted T-cell responses in antitubercular immunity, further studies are required to identify the overall effects of RFP on host resistance against mycobacteria in vivo.
ACKNOWLEDGMENTS
This work was supported by the National Tuberculosis Project (Istituto Superiore di Sanità, Ministero della Sanità, Rome, Italy, [mandato 740, obtained by Grazia Graziani]).
REFERENCES
- 1.Beckman E M, Porcelli S A, Morita C T, Behar S M, Furlong S T, Brenner M B. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg R S, Gerdes D, Chott A, Porcelli S A, Balk S P. Structure and function of the CD1 family of MHC-like cell surface proteins. Immunol Rev. 1995;147:5–29. doi: 10.1111/j.1600-065x.1995.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 3.Bottger E C, Teske A, Kirschner P, Bost S, Chang H R, Beer V, Hirschel B. Disseminated “Mycobacterium genavense” infection in patients with AIDS. Lancet. 1992;340:76–80. doi: 10.1016/0140-6736(92)90397-l. [DOI] [PubMed] [Google Scholar]
- 4.Brogden R N, Fitton A. Rifabutin. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994;47:983–1009. doi: 10.2165/00003495-199447060-00008. [DOI] [PubMed] [Google Scholar]
- 5.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.de Meer G, van Geuns H A. Rising case fatality of bacteriologically proven pulmonary tuberculosis in The Netherlands. Tubercle Lung Dis. 1992;73:83–86. doi: 10.1016/0962-8479(92)90060-w. [DOI] [PubMed] [Google Scholar]
- 7.Dhillon J, Mitchison D A. Influence of BCG-induced immunity on the bacterial activity of isoniazid and rifampicin in experimental tuberculosis of the mouse and guinea-pig. Br J Exp Pathol. 1989;70:103–110. [PMC free article] [PubMed] [Google Scholar]
- 8.Giuliani A, Tentori L, Pepponi R, Porcelli S A, Aquino A, Orlando R, Sugita M, Brenner M B, Bonmassar E, Graziani G. Cytokine-induced expression of CD1b molecules by peripheral blood monocytes: influence of 3′-azido-3′-deoxythymidine. Pharm Res. 1997;35:135–140. doi: 10.1006/phrs.1997.0130. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim M S, Maged Z A, Haron A, Khalil R Y, Attallah A M. Antibiotics and immunity: effect of antibiotics on natural killer, antibody dependent cell-mediated cytotoxicity and antibody production. Chemioterapia. 1987;6:426–430. [PubMed] [Google Scholar]
- 10.Ibrahim M S, Maged Z A, Haron A, Khalil R Y, Attallah A M. Antibiotics and immunity: effect of antibiotics on mitogen responsiveness of lymphocytes and interleukin-2 production. Chemioterapia. 1988;7:369–372. [PubMed] [Google Scholar]
- 11.Kent J H. The epidemiology of multidrug-resistant tuberculosis in the United States. Tuberculosis. 1993;77:1391–1409. doi: 10.1016/s0025-7125(16)30200-0. [DOI] [PubMed] [Google Scholar]
- 12.Kim P K, Kim K S, Lee J S, Jeong H J, Choi I J. –1989. Rifampin therapy in Henoch-Schonlein purpura nephritis accompanied by nephrotic syndrome. Child Nephrol Urol. 1988;9:50–56. [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Liakopoulou A. Effects of rifampin treatment on contact sensitivity reactions to oxazolone in BALB/c mice. Immunopharmacol Immunotoxicol. 1989;11:179–192. doi: 10.3109/08923978909005364. [DOI] [PubMed] [Google Scholar]
- 15.Martin L H, Calabi F, Lefebvre F A, Bilsland C A G, Anmilstein C. Structure and expression of the human thymocyte antigens CD1a, CD1b, and CD1c. Proc Natl Acad Sci USA. 1987;84:9189–9193. doi: 10.1073/pnas.84.24.9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicod L P. Indications for chemoprophylaxis of tuberculosis. Arguments for. Schweiz Med Wochenschr. 1993;123:154–158. [PubMed] [Google Scholar]
- 17.Nightingale S D, Cameron D W, Gordin F M, Sullam P M, Cohon D L, et al. Two controlled trials of rifabutin prophylaxis against Mycobacterium avium complex infection in AIDS. N Engl J Med. 1993;329:828–833. doi: 10.1056/NEJM199309163291202. [DOI] [PubMed] [Google Scholar]
- 18.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1492. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 19.Porcelli S A, Morita C T, Brenner M B. CD1b restricts the response of human CD4− 8− T lymphocytes to a microbial antigen. Nature. 1992;360:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 20.Porcelli S A. The CD1 family: a third lineage of antigen-presenting molecules. Adv Immunol. 1995;59:1–98. doi: 10.1016/s0065-2776(08)60629-x. [DOI] [PubMed] [Google Scholar]
- 21.Prigozy T I, Sieling P A, Clemens D, Stewart P L, Behar S M, Porcelli S A, Brenner M B, Modlin R L, Kroneberg M. The mannose receptor delivers lipoglycan antigen to endosomes for presentation to T cells by CD1b molecules. Immunity. 1997;6:187–197. doi: 10.1016/s1074-7613(00)80425-2. [DOI] [PubMed] [Google Scholar]
- 22.Roszkowski K, Beuth J, Roszkowski W, Jeljaszewicz J, Pulverer G. Influence of 12 antibiotics on antitumor immunity in BALB/c-mice. Int J Med Microbiol Virol Parasitol Infect Dis. 1992;276:280–287. doi: 10.1016/s0934-8840(11)80015-0. [DOI] [PubMed] [Google Scholar]
- 23.Saha B, Das G, Vohra H, Ganguly N K, Mishra G C. Macrophage-T cell interaction in experimental mycobacterial infection. Selective regulation of co-stimulatory molecules on Mycobacterium-infected macrophages and its implication in the suppression of cell-mediated immune response. Eur J Immunol. 1994;24:2618–2624. doi: 10.1002/eji.1830241108. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Schoel B, Gulle H, Kaufmann S H. Heterogeneity of the repertoire of T cells of tuberculosis patients and healthy contacts to Mycobacterium tuberculosis antigens separated by high-resolution techniques. Infect Immun. 1992;60:1717–1725. doi: 10.1128/iai.60.4.1717-1720.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stenger S, Mazzacaro R J, Uyemera K, Cho S, Barnes P F, Rosar J P, Sette A, Brenner M B, Porcelli S A, Bloom B R, Moldin R L. Differential effect of cytolytic subsets on intracellular infection. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 27.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsukaguchi K, Balaji K N, Boom W H. CD4+ αβ T cell and γδ T cell responses to Mycobacterium tuberculosis. J Immunol. 1995;154:1786–1796. [PubMed] [Google Scholar]
- 29.Van Vlem B, Vanholder R, De Paepe P, Vogelaers D, Ringoir S. Immunomodulating effects of antibiotics: literature review. Infection. 1996;24:275–291. doi: 10.1007/BF01743360. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Price C W. Streptolydigin resistance can be conferred by alterations to either the beta or beta′ subunits of Bacillus subtilis RNA polymerase. J Biol Chem. 1995;270:23930–23933. doi: 10.1074/jbc.270.41.23930. [DOI] [PubMed] [Google Scholar]