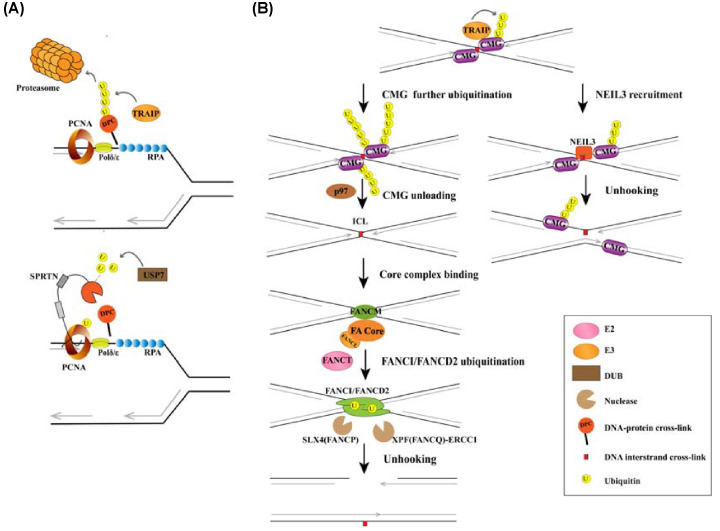
Figure 4. The role of ubiquitination in the removal of DNA-protein cross-links and interstrand cross-links.
(A) The removal of DNA–protein cross-links (DPCs) relies mainly on TRAIP/proteasomes (top), which degrade polyubiquitinated DPCs, or SPRTN (bottom), which is recruited by ubiquitinated PCNA and deubiquinated by USP7. (B) Interstrand cross-link (ICL) removal is mainly mediated by the FA pathway. When two replication forks converge on an ICL, TRAIP-generated Ub chains recruit the glycosylase NEIL3 to directly cleave cross-links, while longer Ub chains promote CDC48/p97-dependent CMG disassembly. Next, a heterotetrameric FANCM complex recognizes the stressed replication forks and recruits the FA core complex. Within the core complex, FANCL (E3 ubiquitin ligase) and FANCT (E2) monoubiquitinate FANCD2 at K561 and FANCI at K523. This step promotes the nuclease-dependent DNA incision by XPF (FANCQ)-ERCC1 and SLX4 (FANCP), leading to the unhooking of ICLs and generation of DSB intermediates.