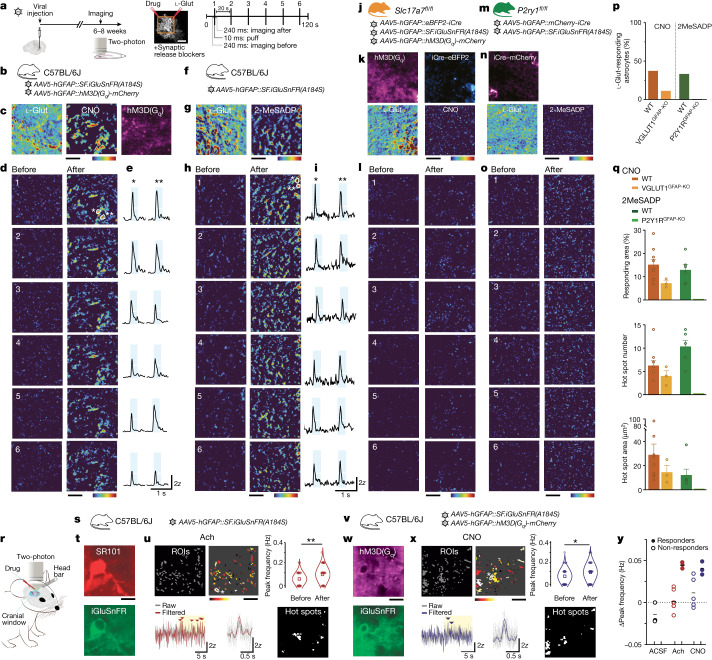
Fig. 2. Fast glutamate secretion at hotspots in a subgroup of astrocytes after selective chemogenetic or endogenous receptor stimulation in situ and in vivo.
a, Schematic of two-photon SF-iGluSnFR glutamate imaging experiments in hippocampal slices from virally injected WT or transgenic mice (details are provided in b,f,j and m) (left). Middle, typical FOV imaged from a DGML astrocyte. Drugs (CNO, 100 µM; 2MeSADP, 10 µM) and l-Glut (1 mM), all in Alexa-594 solution, were locally delivered through two puff pipettes. The slices were incubated with a cocktail of synaptic blockers (Methods). Right, the stimulation protocol used for drug applications. Ten-millisecond puff applications were performed six times, one every 20 s, during 120 s imaging acquisitions. ‘Before’ and ‘after’ correspond to the 240 ms imaging periods before and after each drug application shown in d,h,l and o as individual mean projections of the SF-iGluSnFR signal. Corresponding l-Glut-evoked responses are shown in Extended Data Fig. 4a,b,d,e. The whole-brain image is from the Allen Mouse Brain Connectivity Atlas (https://mouse.brain-map.org/). b–e, SF-iGluSnFR responses to chemogenetic stimulations in a representative astrocyte. b, Experiments in WT mice expressing SF-iGluSnFR and Gq-DREADD (hM3D(Gq)) in DGML astrocytes. c, Mean projection of Gq-DREADD–mCherry expression (right). Middle and left, s.d. projection of SF-iGluSnFR signal variance across 6 CNO (middle) or l-Glut (left) applications (high-variance spots represent repeatedly responding regions, that is, hotspots). d, Individual responses to six CNO applications. e, Traces corresponding to two hotspot regions in d (indicated by asterisks; white line, 2z; azure, 240 ms post-puff period). f–i, SF-iGluSnFR responses to endogenous P2Y1R stimulations in a representative astrocyte. f, Experiments in WT mice expressing SF-iGluSnFR in astrocytes. g, s.d. projection of SF-iGluSnFR signal variance across six applications of the P2Y1R agonist 2MeSADP (right) or l-Glut (left). h, Individual responses to six 2MeSADP applications. i, Traces corresponding to two hot spot regions. Details are as described in e. j–l, Lack of SF-iGluSnFR responses to CNO in a representative astrocyte with deleted VGLUT1 (VGLUT1GFAP-KO). j, Slc17a7fl/fl mice were injected with viral vectors inducing SF-iGluSnFR and Gq-DREADD expression, and iCre-mediated VGLUT1 deletion in triple-fluorescent astrocytes. k, Mean projections of Gq-DREADD–mCherry (top left) and nuclear iCre–eBFP2 (top right) expression, and s.d. projections of SF-iGluSnFR signal variance across six CNO (bottom right) or l-Glut (bottom left) applications. l, Individual responses to six CNO applications. m–o, A lack of SF-iGluSnFR responses to 2MeSADP in a representative astrocyte with deleted P2y1r (P2Y1RGFAP-KO). m, GlastcreERT2P2ry1Rfl/fl mice were injected with viruses to express SF-iGluSnFR and induce iCre-mediated P2y1r deletion in astrocytes. n, Mean projection of iCre–mCherry expression (top) and s.d. projection of SF-iGluSnFR signal variance across six 2MeSADP (bottom right) and l-Glut (bottom left) applications. o, Individual responses to 2MeSADP applications. For c,d,g,h,k,l,n and o, the z-score scale is colour-coded from 0 (dark blue) to 6 (red). p,q, Quantitative analysis of SF-iGluSnFR responses to drugs in DGML astrocytes. p, The proportion of astrocytes responding to (1) CNO in WT (23 cells, 5 mice) and VGLUT1GFAP-KO (24 cells, 5 mice) mice; and (2) 2MeSADP in WT (18 cells, 2 mice) and P2Y1RGFAP-KO (20 cells, 2 mice) mice. All individual cell responses are shown in Extended Data Fig. 6. q, Features of SF-iGluSnFR responses evoked by CNO (WT, n = 9 out of 24 cells; VGLUT1GFAP-KO, n = 3 out of 24 cells) and 2MeSADP (WT, n = 6 out of 18 cells; P2Y1RGLAST-KO, n = 0 out of 20 cells). Top, the percentage of l-Glut-responding FOVs that respond to CNO or to 2MeSADP (the same mouse groups as in p). The number (middle) and area (bottom) of individual hotspots per FOV for CNO and 2MeSADP are shown. r, Schematic of in vivo two-photon SF-iGluSnFR glutamate imaging experiments in the visual cortex of awake mice in the presence of synaptic blockers (details are provided in s and v and the Methods). s–u, SF-iGluSnFR responses to Ach in a representative astrocyte (110 µm below the surface). s, Experiments in WT mice injected with virus to express SF-iGluSnFR in visual cortex astrocytes. t, The red SR-101 signal highlights the astrocyte in the FOV (top). Bottom, cumulative SF-iGluSnFR fluorescence throughout the acquisition from the same astrocyte (n = 8 cells, 3 mice). u, 50 selected ROIs (top left) (Methods), the peak frequency variations of SF-iGluSnFR signal in individual ROIs (colour scale: white (+0.25 Hz) to black (−0.1 Hz)) (top middle) and the mean frequency change in the 50 ROIs (top right) after Ach (10–50 mM) application (Wilcoxon rank-sum test, **P = 0.0059). Bottom left, SF-iGluSnFR traces from a representative ROI (asterisk in the top middle image), before and after (yellow) the Ach puff; the arrowheads indicate SF-iGluSnFR activity peaks. Bottom middle, the averaged kinetics of SF-iGluSnFR events from the bottom left plot, aligned to peak time. Bottom right, hotspot ROIs responding to two Ach applications. v–x, SF-iGluSnFR responses to chemogenetic stimulation in a representative astrocyte (137 µm below the surface). v, Experiments in mice expressing SF-iGluSnFR and Gq-DREADD in visual cortex astrocytes. w, Mean projection of Gq-DREADD–mCherry expression (top). Bottom, cumulative SF-iGluSnFR fluorescence throughout the acquisition from the same astrocyte as in t (n = 11 cells, 3 mice). x, As described in u, but for CNO (0.1–1 mM) infusion. Note the mean frequency change of 50 ROIs after CNO (top right) (Wilcoxon rank-sum test, **P = 0.0282). Bottom right, hotspot ROIs responding to two CNO applications. y, The mean peak frequency changes in SF-iGluSnFR signal after stimulus (Ach, CNO or ACSF) in responding and non-responding astrocytes (individual data are shown in Extended Data Fig. 7g). Scale bars, 10 µm (a,c,d,g,h,k,l,n,o,t,u,w and x).