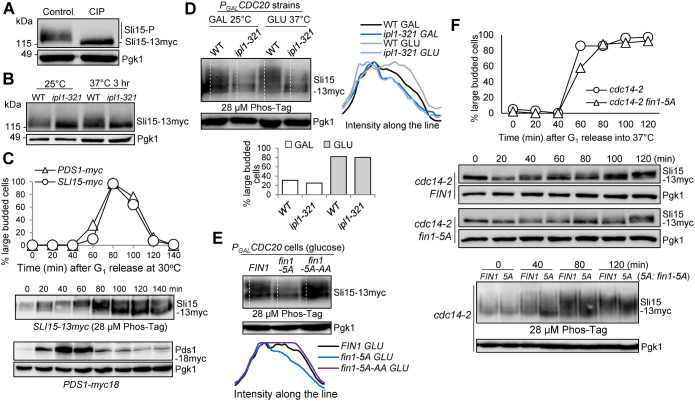
FIGURE 1:
Fin1-PP1 promotes Sli15 phosphorylation. (A) Sli15 is phosphorylated in vivo. Asynchronous WT cells (781-2-2) containing Sli15-13myc were grown in YPD medium at 25°C to log phase. Protein samples were collected and half of the protein sample was treated with calf intestinal alkaline phosphatase (CIP). Western blotting was performed with anti-myc antibody. Pgk1, loading control. (B) Sli15 is phosphorylated by Ipl1 kinase. WT (781-2-2) and ipl1-321 (4175-1-1) cells with Sli15-13myc were grown in YPD medium at 25°C to log phase then shifted to 37°C for 3 h. Protein samples were collected before and after temperature shift. Western blotting was performed with anti-myc antibody. Pgk1, loading control. (C) Sli15 phosphorylation during cell cycle. G1-arrested SLI15-13myc (781-2-2) and PDS1-18myc (JBY649) cells were released into 30°C YPD medium. α-factor was added back after 40-min release to block the following cell cycle. Cells were collected every 20 min to prepare protein samples and to count budding index (N = 100 cells). Phos-tag SDS–PAGE was performed to visualize the bandshift of Sli15 during cell cycle using anti-myc antibody. Regular SDS–PAGE was used to examine Pds1 protein level during cell cycle. Pgk1, loading control. (D) Analyze Sli15 phosphorylation in WT and ipl1-321 cells arrested at metaphase using Phos-tag SDS–PAGE. Asynchronous SLI15-13myc PGALCDC20 (4595-2-1) and ipl1-321 SLI15-13myc PGALCDC20 (4600-2-2) cells were grown overnight in galactose media at 25°C. Glucose was added for Cdc20 depletion and cells were shifted to 37°C at the same time. Cells were collected before and after temperature shift (2 h) to prepare protein samples and to count budding index (N = 100 cells). Phos-tag SDS–PAGE was performed with anti-myc antibody. Pgk1, loading control. Quantifications of the Sli15 protein band-shift (phosphorylation) from the western blot were performed using ImageJ. GLU: glucose; GAL: galactose. (E) Examination of Sli15 dephosphorylation in different fin1 mutants using Phos-tag SDS–PAGE. Asynchronous SLI15-13myc PGALCDC20 (4595-2-1) cells with either FIN1 (pSB1252), phospho-deficient fin1-5A (pSB1359), or phospho- and PP1 binding-deficient fin1-5A-AA (pSB1361) plasmids were grown overnight in galactose media at 30°C. Glucose was added for Cdc20 depletion and metaphase arrest. After 2 h, cells were collected to prepare protein samples and Phos-tag SDS–PAGE was performed to detect Sli15 bandshift with anti-myc antibody. Pgk1, loading control. Quantifications of the Sli15 protein bandshift (phosphorylation) from the Western blot were performed using ImageJ. (F) Premature Fin1-PP1 KT localization promotes Sli15 dephosphorylation in cdc14-2 cells. G1-arrested cdc14-2 SLI15-13myc (4048-1-3) cells containing either FIN1 (pMB6) or fin1-5A (pMB7) plasmids were released into YPD at 37°C. Samples were collected every 20 min to prepare protein samples and to count budding index (N = 100 cells) (top). Regular western blotting was performed to examine Sli15 band shift. Pgk1, loading control. The samples from the indicated time points were also subjected to Phos-tag SDS–PAGE to show Sli15 phosphorylation (bottom). Pgk1, loading control.