Abstract
Macrophage pyroptosis and related inflammatory responses play an important role in periodontitis. Kynurenic acid (KA) is hypothesized to have anti-inflammatory potential, but whether KA can inhibit macrophage pyroptosis and the underlying mechanisms remain unclear. Lipopolysaccharide (LPS) was used to induce pyroptosis in THP-1-derived macrophages. KA or ML385 was used to pretreat macrophages, after which, cell viability, NOD-like receptor protein 3 (NLRP3) inflammasome-related protein expression, oxidative stress levels and nuclear factor erythroid 2-related factor 2 (NRF2) expression were measured. The results showed that KA improved the LPS-induced decrease in macrophage viability and lactate dehydrogenase release. KA prevented THP-1 macrophage pyroptosis induced by LPS by reducing the expression of NLRP3, Gasdermin-D, and Caspase1, and decreased the expression of inflammatory factors. KA suppressed NLRP3 inflammasome activation by inhibiting ROS overproduction and increasing Heme Oxygenase 1 and glutathione levels. Moreover, KA promoted NRF2 translocation from the cytoplasm to the nucleus. In addition, the anti-pyroptotic and antioxidant effects of KA were reversed by ML385 inhibition of NRF2. In the present study, it was found that KA significantly suppressed macrophage pyroptosis induced by LPS. It was further demonstrated that the anti-pyroptotic effects of KA were mediated by activation of the NRF2 pathway.
Keywords: periodontitis, macrophages, kynurenic acid, pyroptosis, oxidative stress
Introduction
Periodontitis is a common chronic inflammatory oral disease. The incidence of periodontitis is increasing annually. Globally, >11% of patients suffer from severe periodontitis (1,2). Bacterial infection of the gums is one of the most common causes of periodontitis (3). In the advanced stages of periodontitis, the invasion of inflammatory cells leads to tooth loss, which seriously affects a patient's quality of life (4). Although drug-based therapy and surgery have seen notable progress, they still possess notable limitations. A previous study reported that adjunctive antimicrobial photodynamic therapy is beneficial to the surgical treatment of periodontitis, but some patients still relapse (5). Furthermore, ultrasonic and hand instrumentation for periodontal therapy reduces the recurrence rate of periodontitis, but increases the risk of secondary infection (6). Therefore, there is a need to explore novel therapeutic methods for the management of periodontitis.
There is increasing evidence suggesting that inflammatory responses contribute to the progression of periodontitis. Macrophages are one of the most common inflammatory cells present in a state of periodontitis (7–9). Previous studies demonstrated that macrophage pyroptosis enhances the development of periodontitis (9,10). Pyroptosis is a type of physiological proinflammatory programmed death. The NOD-like receptor protein 3 (NLRP3) inflammasome pathway is an extensively studied pathway that mediates pyroptosis. As a ubiquitous intracellular receptor, NLRP3 when activated, recruits apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC) and Caspase 1 to form the NLRP3 inflammasome (11,12). Once inflammasome formation has occurred, Gasdermin-D (GSDMD) and inflammatory cytokines are cleaved and activated. Subsequently, GSDMD translocates to the cell membrane and forms holes in the membrane, which leads to the release of inflammatory factors and ultimately pyroptosis (12). It was previously reported that hyperglycemia promotes the progression of diabetes-related periodontitis by inducing macrophage pyroptosis (13). Furthermore, the blockade of macrophage glycolysis can suppress the progression of periodontitis by inhibiting macrophage pyroptosis (14). Together, the results of these previous studies suggest that macrophage pyroptosis may be an effective therapeutic target for the management of periodontitis. However, there remains a lack of effective small-molecule inhibitors to inhibit macrophage pyroptosis.
Kynurenic acid (KA) is one of the metabolites of tryptophan (15). In recent years, KA has been shown to have potential anti-inflammatory properties, and as such has attracted increasing attention. A previous study found that physical exercise may improve inflammatory responses by increasing KA production (16). Furthermore, KA has also been shown to improve mortality in a mouse model of sepsis by inhibiting neutrophil activity and reducing the expression of inflammatory factors (17). However, whether KA can inhibit macrophage pyroptosis and its underlying mechanism remain unclear.
In recent years, oxidative stress has been identified as a powerful activator of NLRP3 (18). It was found that the increased production of reactive oxygen species (ROS) during excessive can be sensed by NLRP3, and this results in activation of the inflammasome, initiating the process of pyroptosis (19). Nuclear factor erythroid 2-related factor 2 (NRF2) is one of the most widely expressed transcription factors (20). NRF2 regulates the expression of various antioxidant proteins and maintains intracellular redox homeostasis by binding to target genes (21,22). Heme Oxygenase 1 (HO-1) and superoxide dismutase (SOD), as classic antioxidant proteins, are also regulated by NRF2. Kelch-like ECH-associated protein 1 (KEAP1) inhibits NRF2 nuclear translocation and leads to ubiquitination degradation by binding to NRF2 (23,24). A KEAP1/NRF2 interaction plays a crucial role in regulating the balance between ROS production and degradation in cells. Previous studies found that exogenous activation of NRF2 inhibits pyroptosis by reducing intracellular ROS levels (25,26). Moreover, it was also found that triptolide, a natural chemical product, can directly induce tumor cell pyroptosis by inhibiting NRF2 activity (27).
In the present study, the effects of KA on THP-1 macrophage pyroptosis induced by lipopolysaccharide (LPS) were investigated. Furthermore, the underlying mechanisms of KA in regulating pyroptosis, and the involvement of the NRF2 pathway were assessed.
Materials and methods
Reagents
KA was obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. (cat. no. 492-27-3). LPS was purchased from MedChemExprss (cat. no. HY-D1056). ML385 was obtained from MedChemExprss (cat. no. 846557-71-9). Phorbol-12-Myristate-13-Acetate (PMA) was purchased from Beyotime Institute of Biotechnology (cat. no. 16561-29-8).
Cell culture
According to a previous study (25), THP-1 cells (ATCC) were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.). A total of 100 nM PMA was used to treat THP-1 cells for 48 h to induce transformation into macrophages. Macrophages were divided into five groups: i) CTRL group (DMSO), ii) LPS group (200 ng/ml LPS for 24 h), iii) LPS/KA group (100 µM KA for 0.5 h, followed by 200 ng/ml LPS for 24 h), iv) ML385/LPS/KA group (5 µM ML385 for 0.5 h, followed by 100 µM KA for 0.5 h, and finally followed by 200 ng/ml LPS for 24 h), v) and ML385 alone group. The macrophages were then used for subsequent experiments.
Western blotting
According to the manufacturer's instructions, RIPA lysis buffer (Beyotime Institute of Biotechnology) was used to extract the total protein from macrophages in the different groups. A BCA protein quantification kit (Beyotime Institute of Biotechnology) was used to measure the protein concentration. Using 10% SDS gels, 40 µg protein was loaded per lane and resolved using SDS-PAGE (80 V for 30 min and 120 V for 100 min). The resolved proteins were transferred to PVDF membranes (MilliporeSigma) using a semidry transblot apparatus (Bio-Rad Laboratories, Inc.; transfer conditions: Constant voltage, 15 V for 30 min). Next, the membranes were blocked using 5% skimmed milk at room temperature for 60 min, washed 3 times with PBS for 10 min each, and subsequently incubated with the primary antibodies followed by the secondary antibody. The primary antibodies used were: NLRP3 (cat. no. ab263899; 1:1,000), GSDMD (cat. no. ab210070; 1:1,000), caspase-1 (cat. no. ab179515; 1:1,000), NRF2 (cat. no. ab137550; 1:1,000), and HO-1 (cat. no. ab52947; 1:1,000) (all Abcam). Between the incubations with the primary and secondary antibody incubations, the membranes were washed three times with TBS-T (0.1% Tween; 10 min per wash). Membranes were incubated with the secondary antibody at room temperature for 1 h. The secondary antibody used was an HRP-labeled Goat Anti-Rabbit IgG (H+L) (cat. no. A0208; 1:10,000; Beyotime Institute of Biotechnology). Finally, the membrane was washed three times with TBS-T (10 min per wash), and signals were visualized using an enhanced chemiluminescence reagent (Beijing Solarbio Science & Technology Co., Ltd.). ImageJ version 1.4 (National Institutes of Health) was used for densitometry analysis.
Hoechst/propidium iodide (PI) staining
The macrophages were seeded into 48-well plates with 20,000 cells per well. After the macrophages were treated, the cells were washed three times with cold PBS at room temperature. Then, 5 µl Hoechst staining solution was added, followed by 5 µl PI stain. The macrophages were mixed and incubated in an ice bath at 4°C for 25 min and subsequently washed three times with PBS, and an anti-fluorescence quenching agent (Beyotime Institute of Biotechnology) was added. Macrophages were observed under a fluorescence microscope at ×200 magnification and images were captured. To calculate the percentage of PI-positive cells, the number of PI-positive cells was divided by the total number of Hoechst-positive cells; three random fields were chosen for this calculation.
Macrophage viability assays
Macrophages were cultured in 96-well plates (10,000 cells per well). The Cell counting Kit-8 (CCK-8) (Beyotime Institute of Biotechnology) working solution was prepared in 1:10 dilution using the medium, and 100 µl working solution was added to each well and incubated at 37°C for 1 h. After incubation, the absorbance was measured at 450 nm using a microplate spectrophotometer (Tecan Group, Ltd.).
Malondialdehyde (MDA), glutathione (GSH), and superoxide dismutase (SOD) measurement
A total of 1×106 macrophages were plated in 6-well plates and treated as above. The lysates of macrophages were extracted according to the manufacturer's instructions, and the levels of MDA (cat. no. S0131S; Beyotime Institute of Biotechnology), GSH (cat. no. S0053; Beyotime Institute of Biotechnology) and SOD (cat. no. S0101S; Beyotime Institute of Biotechnology) were measured using specific kits, and the absorbance (MDA, 532 nm; GSH, 412 nm; SOD, 450 nm) was analyzed using a microplate spectrophotometer (Tecan Group, Ltd.).
Lactate dehydrogenase (LDH) assays
Macrophages were cultured in 96 well plates with 10,000 cells per well. A total of 80 µl cell supernatant was collected and centrifuged (400 × g, 4°C for 10 min). According to the manufacturer's protocol (cat. no. C0016; Beyotime Institute of Biotechnology), i) the macrophage supernatant was aspirated; ii) 150 µl diluted LDH-releasing reagent was added; iii) the plates were shaken and incubated at 37°C for 1 h; iv) the culture plate was removed and centrifuged (400 × g, 4°C for 5 min), and 120 µl supernatant was collected. A total of 120 µl of supernatant was added to a new 96-well plate separately. Thereafter the absorbance was measured at 490 nm.
Cell nuclei isolation
The Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime Institute of Biotechnology) was used to isolate the cell nuclei. The experimental procedures were performed according to the manufacturer's protocol. Finally, the supernatant containing the nuclear proteins was collected.
Intracellular ROS assay
Macrophages were cultured in 96 well plates with 10,000 cells per well. The macrophages were treated under different conditions. According to the 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) (cat. no. S0033S; Beyotime Institute of Biotechnology) instructions, i) DCFH-DA was diluted 1,000× using PBS; ii) 100 µl working solution was added into the cells and cells were incubated at 37°C for 20 min and washed three times with PBS (5 min per wash); and iii) ROS intensity was observed under a fluorescence microscope (×200 magnification, excitation wavelength of 488 nm and emission wavelength of 525 nm).
ELISA
Macrophages were cultured in 96 well plates with 10,000 cells per well and treated as above. A total of 80 µl cell supernatant was collected and stored at −80°C. The levels of IL-1β, IL-18, and TNF-α were measured according to the manufacturer's instructions (TNF-α, cat. no. ab181421; IL-1β, cat. no. ab214025; IL-18, cat. no. ab215539; all from Abcam).
Immunofluorescence
Macrophages were cultured in 24 well plates with 50,000 cells per well. Cells were fixed using 4% paraformaldehyde at room temperature for 10 min. The cells were carefully washed with PBS at room temperature three times (5 min per wash), after which they were treated with 0.3% Triton X at room temperature for 5 min to permeabilize the cells. Cells were carefully washed again as above, and subsequently blocked with 5% bovine serum albumin for 60 min at room temperature. After washing again, the macrophages were incubated with the primary antibody at 4°C for 12 h. The primary antibody used was an anti-NRF2 (cat. no. 12721; 1:200; Cell signaling Technology, Inc.), and the secondary antibody used was a fluorescent Goat Anti-Rabbit secondary antibody (cat. no. ab150077; 1:500; Abcam) for 2 h at room temperature. After washing, cells were counterstained with 200 µl DAPI at room temperature for 10 min. The macrophages were observed, and images were captured on a confocal laser microscope (LSM 800) at a magnification of ×640.
Statistical analysis
All statistical analysis was performed using GraphPad Prism version 8.0 (GraphPad Software, Inc.). Data are presented as the mean ± SD of three independent experiments. Statistical differences between groups were determined using an unpaired Student's t-test or a one-way ANOVA followed by a Tukey's post hoc analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
KA attenuates macrophage pyroptosis induced by LPS
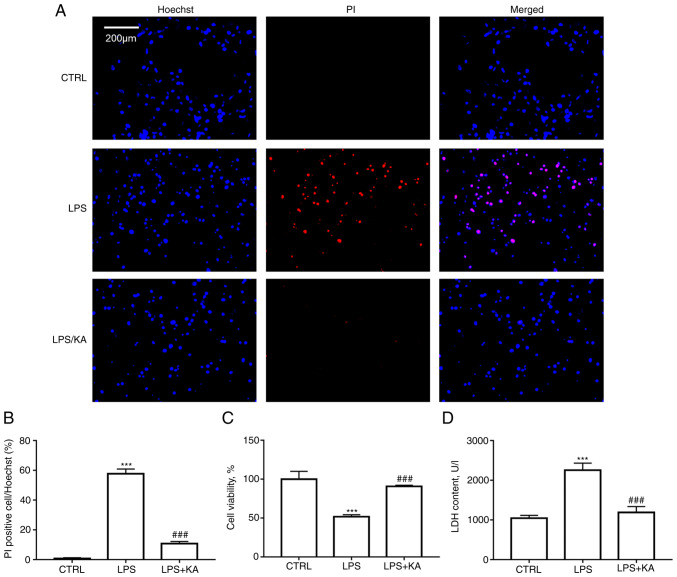
To determine the optimal concentration of KA for use in subsequent experiments, macrophages were treated with 0, 10, 20, 50, 100, 200, or 500 µM KA. A CCK-8 assay showed that KA concentrations lower than 100 µM had no significant inhibitory effect on macrophage activity (Fig. S1). Therefore, 100 µM KA was used to pretreat macrophages in the subsequent experiment. Hoechst-PI staining was performed to analyze the protective effect of KA on macrophage pyroptosis induced by LPS. The results indicated that KA significantly reduced the levels of PI-positive cells following LPS treatment (Fig. 1A and B). CCK-8 assays also showed that KA pretreatment significantly improved cell viability compared with the LPS alone group (Fig. 1C). Intracellular LDH release is a key characteristic of pyroptosis. It was observed that KA pretreatment significantly reduced LDH levels in the cell supernatant of the LPS group (Fig. 1D). These data demonstrated that KA inhibited macrophage pyroptosis induced by LPS.
Figure 1.
KA ameliorates the decrease in cell viability induced by LPS. (A and B) Hoechst PI staining. Macrophages were divided into three groups. CTRL group; LPS group, macrophages were treated with 200 ng/ml LPS for 24 h, and the KA/LPS group, macrophages were treated with 100 µM KA for 0.5 h followed by 200 ng/ml LPS for 24 h. (C) CCK-8 assay. (D) LDH assay. ***P<0.001 vs. CTRL group. ###P<0.001 vs. LPS group. n=3 per group. KA, Kynurenic acid; LPS, lipopolysaccharide; PI, propidium iodide.
KA inhibits inflammatory responses and NLRP3 inflammasome activation induced by LPS
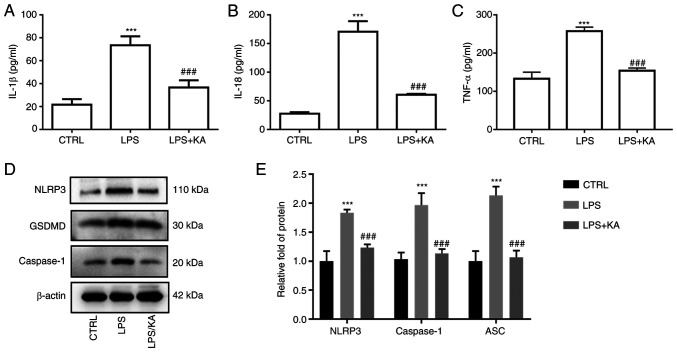
To evaluate the anti-inflammatory effect of KA in macrophages, the levels of IL-1β, IL-18, and TNF-α in the cell supernatant were measured. The results showed that KA significantly reduced the IL-1β, IL-18, and TNF-α levels in the LPS group (Fig. 2A-C). Activation of the NLRP3 inflammasome is one of the most important mechanisms of pyroptosis. To confirm the role of LPS-induced pyroptosis in macrophages, macrophages were pretreated with VX-765, an inhibitor of Caspase1. Both the decrease in cell viability and the increase in LDH levels in the LPS group were attenuated by VX-765 (Fig. S2A and B). Furthermore, VX-765 also decreased the GSDMD levels (Fig. S2C and D). Next, the levels of NLRP3, Caspase1, and GSDMD in macrophages were measured, and it was found that the expression levels of all three were increased in the LPS group, and KA pretreatment notably reduced their expression (Fig. 2D and E). Of note, the expression of NLRP3, Caspase1, and GSDMD was not affected by KA alone (Fig. S3). These data suggested that KA significantly inhibited the inflammatory response and activation of inflammatory bodies induced by LPS.
Figure 2.
KA decreases the levels of NLRP3, Caspase1, and GSDMD. Macrophages were divided into three groups. CTRL group; LPS group, macrophages were treated with 200 ng/ml LPS for 24 h, and the KA/LPS group, macrophages were treated with 100 µM KA for 0.5 h followed by 200 ng/ml LPS for 24 h, after which the levels of inflammatory cytokines in the cell supernatant were detected. (A) IL-1β, (B) IL-18, (C) TNF-α, and (D and E) the protein expression levels of NLRP3, Caspase1, and GSDMD in macrophages. ***P<0.001 vs. CTRL group. ###P<0.001 vs. LPS group. n=3 per group. KA, Kynurenic acid; LPS, lipopolysaccharide; PI, propidium iodide; NLRP3, NOD-like receptor protein 3; GSDMD, Gasdermin-D.
KA reduces oxidative stress in macrophages induced by LPS
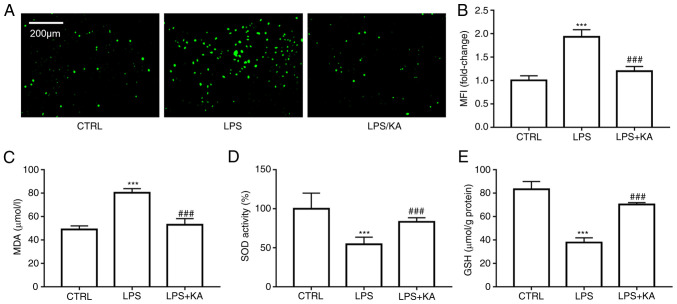
Oxidative stress is an effective activator of the NLRP3 inflammasome. To confirm the effect of KA on the production of ROS, DCFH-DA staining was used to analyze the intracellular ROS levels. KA pretreatment significantly decreased the ROS levels in macrophages (Fig. 3A and B). Malondialdehyde (MDA) is a metabolite of lipid peroxidation in cell membranes, MDA levels represent the levels of lipid peroxidation in cells. It was found that KA decreased the MDA levels in the LPS group (Fig. 3C). Furthermore, KA increased the GSH levels and SOD activity (Fig. 3D and E). These data showed that KA improved oxidative stress levels after LPS treatment by reducing ROS production and increasing antioxidant protein activity.
Figure 3.
KA inhibits oxidative stress in macrophages induced by LPS. The macrophages were divided Macrophages were divided into three groups. CTRL group; LPS group, macrophages were treated with 200 ng/ml LPS for 24 h, and the KA/LPS group, macrophages were treated with 100 µM KA for 0.5 h followed by 200 ng/ml LPS for 24 h (A and B) DCFH-DA was used to analyze the total ROS levels. (C) MDA levels, (D) SOD levels, and (E) GSH levels in the different group. ***P<0.001 vs. CTRL group. ###P<0.001 vs. LPS group. n=3 per group. KA, Kynurenic acid; LPS, lipopolysaccharide; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione; ROS, reactive oxygen species.
KA increases the activity of NRF2 in macrophages
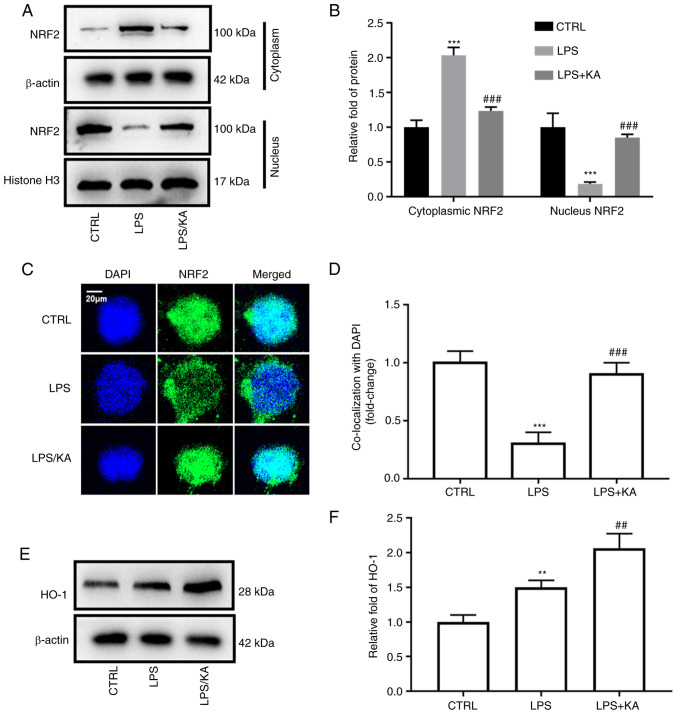
NRF2 is an important transcription factor for the maintenance of cellular oxidative stress homeostasis. Cytoplasmic and nuclear proteins were extracted from the different groups and the expression of NRF2 was assessed. KA decreased NRF2 levels in the cytoplasm, but increased NRF2 levels in the nucleus (Fig. 4A and B). The total NRF2 levels in macrophages were not altered in the three groups (Fig. S4). Furthermore, immunofluorescence analysis also showed that KA increased the levels of NRF2 in the nucleus (Fig. 4C and D). HO-1 is a downstream protein of NRF2. After activation of NRF2, it enters the nucleus and binds to the promoter of HO-1. Here, it was found that LPS increased the HO-1 levels, and KA further increased HO-1 levels in the LPS group (Fig. 4E and F). These data demonstrated that KA activates the NRF2 pathway by promoting NRF2 translocation.
Figure 4.
KA enhances NRF2 nuclear translocation. Macrophages were divided into three groups. CTRL group; LPS group, macrophages were treated with 200 ng/ml LPS for 24 h, and the KA/LPS group, macrophages were treated with 100 µM KA for 0.5 h followed by 200 ng/ml LPS for 24 h. (A and B) Macrophage nuclear and cytoplasmic proteins were extracted and western blotting was used to evaluate the levels of NRF2. (C and D) Immunofluorescence was used to observe the levels of NRF2 in the nuclei of different groups. (E and F) The levels of HO-1 in macrophages. **P<0.01, ***P<0.001 vs. CTRL group. ##P<0.01, ###P<0.001 vs. LPS. n=3 per group. KA, Kynurenic acid; LPS, lipopolysaccharide; NRF2, Nuclear factor erythroid 2-related factor 2; HO-1, Heme-oxygenase-1.
KA improves oxidative stress by activating the NRF2 pathway
To confirm whether KA decreased ROS levels by activating the NRF2 pathway. ML385, a specific inhibitor for NRF2, was used to pretreat macrophages. It was found that ML385 significantly increased the ROS levels in KA-pretreated macrophages (Fig. 5A and B). It was also found that ML385 augmented the decrease in MDA levels in KA + ML385 treated macrophages (Fig. 5C). Moreover, the increase in GSH levels, SOD activity, and HO-1 levels in the KA group was reduced following ML385 pretreatment (Fig. 5D-G). These data demonstrated that the anti-oxidative effects of KA were abrogated by ML385.
Figure 5.
ML385 abrogates the antioxidant effects of KA. The macrophages were divided into five groups: CTRL group; LPS group, macrophages were treated with 200 ng/ml LPS for 24 h; KA/LPS group, macrophages were treated with 100 µM KA for 0.5 h followed by 200 ng/ml LPS for 24 h; LPS/KA/ML385 group, macrophages were treated with 5 µM ML385 for 0.5 h, 100 µM KA for 0.5 h, followed by 200 ng/ml LPS for 24 h; and ML385 alone group, macrophages were treated with 5 µM ML385 for 24 h. (A and B) DCFH-DA was used to analyze the total ROS levels. (C) MDA, (D) SOD, and (E) GSH levels in the different group. (F and G) HO-1 levels in the different groups. ***P<0.001 vs. CTRL group; #P<0.05, ##P<0.01, ###P<0.001 vs. LPS group; &&&P<0.001 vs. LPS/KA group. n=3 per group. KA, Kynurenic acid; LPS, lipopolysaccharide; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione; ROS, reactive oxygen species; HO-1, Heme-oxygenase-1.
KA inhibits macrophage pyroptosis by activating the NRF2 pathway
Excessive ROS production is a key mechanism in the activation of the NLRP3 inflammasome. The results suggested that KA reduced oxidative stress by activating NRF2. However, whether KA inhibits macrophage pyroptosis via regulation of the NRF2 pathway remains unclear. Hoechst/PI staining and CCK-8 assays showed that ML385 abrogated the protective effects of KA on macrophages (Fig. 6A-C). Moreover, following ML385 treatment, the levels of LDH in the supernatant of the KA group were also significantly increased (Fig. 6D). It was observed that the decrease in NLRP3, Caspase1, and GSDMD levels in the KA group was augmented following ML385 pretreatment (Fig. 6E and F). Furthermore, compared to the KA + ML385 combined group, the levels of IL-1β, IL-18, and TNF-α in the cell supernatant were also increased after ML385 pretreatment (Fig. 6G-I). These data showed that KA inhibited macrophage pyroptosis induced by LPS via activation of the NRF2 pathway.
Figure 6.
The macrophages were divided into five groups: CTRL group; LPS group, macrophages were treated with 200 ng/ml LPS for 24 h; KA/LPS group, macrophages were treated with 100 µM KA for 0.5 h followed by 200 ng/ml LPS for 24 h; LPS/KA/ML385 group, macrophages were treated with 5 µM ML385 for 0.5 h, 100 µM KA for 0.5 h, followed by 200 ng/ml LPS for 24 h; and ML385 alone group, macrophages were treated with 5 µM ML385 for 24 h. (A and B) Hoechst-PI staining. (C) CCK-8 assay. (D) LDH assay. (E and F) The protein expression levels of NLRP3, Caspase1, and GSDMD in macrophages. The levels of inflammatory cytokines in the cell supernatant were detected. (G) IL-1β, (H) IL-18, and (I) TNF-α. ***P<0.001 vs. CTRL group; ###P<0.001 vs. LPS group; &&&P<0.001 vs. LPS/KA group. n=3 per group. KA, Kynurenic acid; LPS, lipopolysaccharide; PI, propidium iodide; LDH, lactate dehydrogenase; NLRP3, NOD-like receptor protein 3; GSDMD, Gasdermin-D.
Discussion
Periodontitis is one of the most common chronic inflammatory diseases, and is also a common cause of tooth loss. Macrophage-mediated inflammatory responses are hypothesized to play an important role in the progression of periodontitis, the inhibition of macrophage inflammatory responses may thus be a potential interventional target (28). In the present study, it was found that KA inhibited macrophage pyroptosis and increased the inflammatory response induced by LPS. Furthermore, it was also demonstrated that KA attenuated macrophage pyroptosis through activation of the NRF2 pathway.
Oral bacteria-mediated inflammatory responses are an important pathological mechanism of periodontitis (29,30). Previous studies reported that macrophage infiltration has been widely observed in the gums of patients with periodontitis (31,32). The secretion of matrix metalloproteinases by macrophages leads to the degradation of gum tissue, which is one of the key mechanisms of tooth loss (33). Moreover, the infiltrating macrophages undergo apoptosis, which further aggravates the local inflammatory response of the gum (34). It was previously reported that hyperglycemia promotes ligature-induced periodontitis by modulating M1/M2 macrophage polarization via ROS overproduction (35). Therefore, improving the inflammation of macrophages may be a potential treatment for periodontitis. It was found that quercetin improves periodontitis by inhibiting M1 polarization in macrophages (36). However, there is still a lack of endogenous molecules that inhibit macrophage inflammation levels in periodontitis. KA is a metabolite of tryptophan, a common amino acid in humans. A previous study showed that KA may be a key mediator by which physical exercise reduces inflammation levels (12). However, there is evidence directly confirming the anti-inflammatory effect of KA is lacking. In the present study, we found that 100 µM KA decreased the levels of IL-1β, IL-18, and TNF-α levels in macrophages. This result strongly supports the conclusion of previous studies on the anti-inflammatory potential of KA.
Pyroptosis is a relatively recently discovered form of proinflammatory programmed cell death. The primary feature of pyroptosis is the release of several inflammatory factors after the formation of a cell membrane cavity. Previous studies have focused on the role of macrophage pyroptosis in periodontitis (35,37). It was reported that hyperglycemia exacerbates the progression of periodontitis by promoting macrophage pyroptosis (13). It was also found that miR-223-3p regulates pyroptosis through an NLRP3-Caspase1-GSDMD signal axis in periodontitis (38). Furthermore, there is also evidence that macrophage pyroptosis may be a potential cause of inflammatory responses in periodontitis (39,40). Here, it was found that KA significantly improved the LPS-induced decrease in macrophage viability. The cell membrane was perforated after pyroptosis, resulting in the release of LDH and positive PI staining. LPS-induced LDH release levels and the percentage of PI staining positive decreased significantly after KA pretreatment. These data suggested that KA inhibited macrophage pyroptosis. Moreover, KA decreased the levels of NLRP3, Caspase1, and GSDMD in macrophages. These results suggested that KA treatment inhibited the NLRP3 inflammasome activation in macrophages induced by LPS. NLRP3 inflammasome activation is regarded as the typical mechanism of pyroptosis. The NLRP3 inflammasome is assembled of NLRP3 caspase, and ASC, and leads to the cleavage and activation of Caspase1 and GSDMD (41). Therefore, the results of the present study demonstrated KA inhibited macrophage pyrosis by inhibiting classical NLRP3 inflammasome activation.
Since the vital role of the NLRP3 inflammasome in inflammation-related diseases was discovered, the underlying mechanism of NLRP3 inflammasome activation has received extensive attention. Previous studies have confirmed the effect of ROS in activating the NLRP3 inflammasome (42,43). It was found that nicotine activates the NLRP3 inflammasome by increasing ROS levels leading to pyroptosis in endothelial cells (44). Moreover, Previous studies also found that LPS caused macrophage pyroptosis by increasing intracellular ROS levels (25,26). Quercetin, a natural compound, has also been found to suppress macrophage pyroptosis by inhibiting ROS production (25,26). In the present study, it was found that the intracellular ROS levels were increased in macrophages treated with LPS, while KA pretreatment attenuated this. It was also found that KA decreased the MDA levels in LPS-treated macrophages. Furthermore, it was observed that KA increased the activity of the antioxidant system in macrophages, in line with the increase in the levels of GSH, HO-1, and SOD activity. However, LPS alone reduced the expression of nuclear NRF2 but increased the levels of HO-1. Similar results have been reported in previous studies (25,45). There may be several reasons for this result. First, in addition to NRF2, HO-1 is regulated by a variety of transcription factors (46). Secondly, redox imbalances occur after LPS treatment. The total negative feedback regulation of cells leads to the increase of HO-1.
Next the potential mechanism by KA improved oxidative stress was assessed. As a classical transcription factor, NRF2 has been reported to regulate the transcription of several antioxidant proteins, including HO-1. NRF2 expression increased in the cytoplasm and decreased in the nucleus after LPS treatment. However, the effect was reversed by KA, and the HO-1 levels also increased significantly. The results demonstrated that KA may ameliorate oxidative stress by promoting NRF2 nuclear translocation. To further confirm this hypothesis, macrophages were pretreated with ML385, an inhibitor of NRF2 nuclear translocation. ML385 notably abolished the antioxidant stress effect of KA. Furthermore, both the increase in macrophage viability and the reduced activity of the NLRP3 inflammasome caused by KA were abolished by ML385. These results strongly support the notion that KA reduced oxidative stress and pyroptosis by activating the NRF2 pathway. Previous studies have reported the effect of exogenous activation of NRF2 and the protection this imbues against pyroptosis (25,26). Eldecalcitol was found to inhibit LPS-induced pyroptosis of human gingival fibroblasts by activating the NRF2/HO-1 pathway (47). Melatonin also ameliorates LPS-induced pyroptosis of lung epithelial cells by activating the NRF2 pathway (48). The results of the present study suggest that KA may inhibit the progression of periodontitis by improving macrophage pyroptosis. However, this conclusion needs to be confirmed in vivo. A previous study reported that inhibiting NRF2 activity using triptolide reduced tumor cell pyroptosis directly (27), whereas here it was found that ML385 alone did not affect the level of NLRP3, Caspase1, and GSDMD. This difference may be due to the fact that triptolide is a monomeric compound, and the intensity and mechanism of NRF2 inhibition activity of triptolide and ML385 may differ. Secondly, Cai et al (27) used tumor cells to investigate NRF2 activity and the role of pyroptosis, while macrophages were used in the present study. Thus, the difference in cell lines may partly explain the difference in results. Thirdly, it has been reported in several studies that treatment with 5 µM ML385 alone does not directly cause macrophage pyroptosis (25,49).
In the present study, the protective effect of KA against LPS-induced pyroptosis of macrophages in vitro was assessed. However, these data do not directly support whether KA protects against periodontitis by inhibiting macrophage pyroptosis. This is one of the limitations of the present study, and subsequent work is required to determine the effect and underlying mechanism of KA on periodontitis in animal models. Furthermore, VX765 was used as a positive control in the present study, which was used to confirm that LPS can induce macrophage pyroptosis. However, the mechanism by which VX765 inhibits pyroptosis differs from that of KA, thus, VX765 and KA were not used in all experiments.
In conclusion, in the present study, it was found that KA significantly suppressed macrophage pyroptosis induced by LPS. We further demonstrated that the anti-pyroptotic effect of KA was achieved through activation of the NRF2 pathway.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
The present study was funded by the Heilongjiang Province Applied Technology Research and Development Program (grant no. GA17C011) and Jiamusi University Youth Innovative Talent Training Support Program (grant no. JMSUQP2023030).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YG and JD performed the experiments, analyzed the data, and wrote the manuscript. XG and CL analyzed the data. XG, LZ, and SS performed the experiments. YZ, YL and SW designed the study and performed the majority of the experiments. YG and JD confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Slots J. Periodontitis: Facts, fallacies and the future. Periodontol. 2017;75:7–23. doi: 10.1111/prd.12221. [DOI] [PubMed] [Google Scholar]
- 2.Teles F, Collman RG, Mominkhan D, Wang Y. Viruses, periodontitis, and comorbidities. Periodontol. 2022;89:190–206. doi: 10.1111/prd.12435. [DOI] [PubMed] [Google Scholar]
- 3.Nagasawa Y, Misaki T, Ito S, Naka S, Wato K, Nomura R, Matsumoto-Nakano M, Nakano K. Title IgA nephropathy and oral bacterial species related to dental caries and periodontitis. Int J Mol Sci. 2022;23:725. doi: 10.3390/ijms23020725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suvan J, Leira Y, Sancho FM, Graziani F, Derks J, Tomasi C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J Clin Periodontol. 2020;22:155–175. doi: 10.1111/jcpe.13245. [DOI] [PubMed] [Google Scholar]
- 5.Haas AN, Furlaneto F, Gaio EJ, Gomes SC, Palioto DB, Castilho RM, Sanz M, Messora MR. New tendencies in non-surgical periodontal therapy. Braz Oral Res. 2021;35:e095. doi: 10.1590/1807-3107bor-2021.vol35.0095. [DOI] [PubMed] [Google Scholar]
- 6.Krishna R, De Stefano JA. Ultrasonic vs. hand instrumentation in periodontal therapy: Clinical outcomes. Periodontol. 2016;71:113–127. doi: 10.1111/prd.12119. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Ling J, Jiang Q. Inflammasomes in alveolar bone loss. Front Immunol. 2021;12:691013. doi: 10.3389/fimmu.2021.691013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Wang Z, He Y, Peng L, Zhu J, Zhang X. Pyroptosis may play a crucial role in modifications of the immune microenvironment in periodontitis. J Periodontal Res. 2022;57:977–990. doi: 10.1111/jre.13035. [DOI] [PubMed] [Google Scholar]
- 9.Ning W, Acharya A, Li S, Schmalz G, Huang S. Identification of key pyroptosis-related genes and distinct pyroptosis-related clusters in periodontitis. Front Immunol. 2022;13:862049. doi: 10.3389/fimmu.2022.862049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun X, Gao J, Meng X, Lu X, Zhang L, Chen R. Polarized macrophages in periodontitis: Characteristics, function, and molecular signaling. Front Immunol. 2021;12:763334. doi: 10.3389/fimmu.2021.763334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22:550–559. doi: 10.1038/s41590-021-00886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooftman A, Angiari S, Hester S, Corcoran SE, Runtsch MC, Ling C, Ruzek MC, Slivka PF, McGettrick AF, Banahan K, et al. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metab. 2020;32:468–478.e7. doi: 10.1016/j.cmet.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao P, Yue Z, Nie L, Zhao Z, Wang Q, Chen J, Wang Q. Hyperglycaemia-associated macrophage pyroptosis accelerates periodontal inflamm-aging. J Clin Periodontol. 2021;48:1379–1392. doi: 10.1111/jcpe.13517. [DOI] [PubMed] [Google Scholar]
- 14.He Y, Wang Y, Jia X, Li Y, Yang Y, Pan L, Zhao R, Han Y, Wang F, Guan X, Hou T. Glycolytic reprogramming controls periodontitis-associated macrophage pyroptosis via AMPK/SIRT1/NF-κB signaling pathway. Int Immunopharmacol. 2023;119:110192. doi: 10.1016/j.intimp.2023.110192. [DOI] [PubMed] [Google Scholar]
- 15.Tóth F, Cseh EK, Vécsei L. Natural molecules and neuroprotection: Kynurenic acid, pantethine and α-lipoic acid. Int J Mol Sci. 2021;22:403. doi: 10.3390/ijms22010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agudelo LZ, Ferreira DMS, Cervenka I, Bryzgalova G, Dadvar S, Jannig PR, Pettersson-Klein AT, Lakshmikanth T, Sustarsic EG, Porsmyr-Palmertz M, et al. Kynurenic acid and Gpr35 regulate adipose tissue energy homeostasis and inflammation. Cell Metab. 2018;27:378–392.e5. doi: 10.1016/j.cmet.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Ishida Y, Fujita H, Aratani S, Chijiiwa M, Taniguchi N, Yokota M, Ogihara Y, Uoshima N, Nagashima F, Uchino H, Nakajima T. The NRF2-PGC-1β pathway activates kynurenine aminotransferase 4 via attenuation of an E3 ubiquitin ligase, synoviolin, in a cecal ligation/perforation-induced septic mouse model. Mol Med Rep. 2018;18:2467–2475. doi: 10.3892/mmr.2018.9175. [DOI] [PubMed] [Google Scholar]
- 18.An Y, Zhang H, Wang C, Jiao F, Xu H, Wang X, Luan W, Ma F, Ni L, Tang X, et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. 2019;33:12515–12527. doi: 10.1096/fj.201802805RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Q, Li S, Jiang N, Shao X, Zhang M, Jin H, Zhang Z, Shen J, Zhou Y, Zhou W, et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019;26:101254. doi: 10.1016/j.redox.2019.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He F, Ru X, Wen T. NRF2, a transcription factor for stress response and beyond. Int J Mol Sci. 2020;21:4777. doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He F, Antonucci L, Karin M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis. 2020;41:405–416. doi: 10.1093/carcin/bgaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Ulasov AV, Rosenkranz AA, Georgiev GP, Sobolev AS. Nrf2/Keap1/ARE signaling: Towards specific regulation. Life Sci. 2022;291:120111. doi: 10.1016/j.lfs.2021.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo X, Weng X, Bao X, Bai X, Lv Y, Zhang S, Chen Y, Zhao C, Zeng M, Huang J, et al. A novel anti-atherosclerotic mechanism of quercetin: Competitive binding to KEAP1 via Arg483 to inhibit macrophage pyroptosis. Redox Biol. 2022;57:102511. doi: 10.1016/j.redox.2022.102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo X, Bao X, Weng X, Bai X, Feng Y, Huang J, Liu S, Jia H, Yu B. The protective effect of quercetin on macrophage pyroptosis via TLR2/Myd88/NF-κB and ROS/AMPK pathway. Life Sci. 2022;291:120064. doi: 10.1016/j.lfs.2021.120064. [DOI] [PubMed] [Google Scholar]
- 27.Cai J, Yi M, Tan Y, Li X, Li G, Zeng Z, Xiong W, Xiang B. Natural product triptolide induces GSDME-mediated pyroptosis in head and neck cancer through suppressing mitochondrial hexokinase-II. J Exp Clin Cancer Res. 2021;40:190. doi: 10.1186/s13046-021-02100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon T, Lamster IB, Levin L. Current concepts in the management of periodontitis. Int Dent J. 2021;71:462–476. doi: 10.1111/idj.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mombelli A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol. 2018;76:85–96. doi: 10.1111/prd.12147. [DOI] [PubMed] [Google Scholar]
- 30.Sztukowska MN, Roky M, Demuth DR. Peptide and non-peptide mimetics as potential therapeutics targeting oral bacteria and oral biofilms. Mol Oral Microbiol. 2019;34:169–182. doi: 10.1111/omi.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, Zheng C, Yang J, Li B. Intersection between macrophages and periodontal pathogens in periodontitis. J Leukoc Biol. 2021;110:577–583. doi: 10.1002/JLB.4MR0421-756R. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang Z, Yoshizawa-Smith S, Glowacki A, Maltos K, Pacheco C, Shehabeldin M, Mulkeen M, Myers N, Chong R, Verdelis K, et al. Induction of M2 macrophages prevents bone loss in murine periodontitis models. J Dent Res. 2019;98:200–208. doi: 10.1177/0022034518805984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Li X, Yan H, Huang L. Salivary matrix metalloproteinase (MMP)-8 as a biomarker for periodontitis: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e9642. doi: 10.1097/MD.0000000000009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luchian I, Goriuc A, Sandu D, Covasa M. The role of matrix metalloproteinases (MMP-8, MMP-9, MMP-13) in periodontal and peri-implant pathological processes. Int J Mol Sci. 2022;23:1806. doi: 10.3390/ijms23031806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang B, Yang Y, Yi J, Zhao Z, Ye R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J Periodontal Res. 2021;56:991–1005. doi: 10.1111/jre.12912. [DOI] [PubMed] [Google Scholar]
- 36.Meng Q, Li Y, Ji T, Chao Y, Li J, Fu Y, Wang S, Chen Q, Chen W, Huang F, et al. Estrogen prevent atherosclerosis by attenuating endothelial cell pyroptosis via activation of estrogen receptor α-mediated autophagy. J Adv Res. 2021;28:149–164. doi: 10.1016/j.jare.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almubarak A, Tanagala KKK, Papapanou PN, Lalla E, Momen-Heravi F. Disruption of monocyte and macrophage homeostasis in periodontitis. Front Immunol. 2020;11:330. doi: 10.3389/fimmu.2020.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia Y, Zhou K, Sun M, Shu R, Qian J, Xie Y. The miR-223-3p regulates pyroptosis through NLRP3-caspase 1-GSDMD signal axis in periodontitis. Inflammation. 2021;44:2531–2542. doi: 10.1007/s10753-021-01522-y. [DOI] [PubMed] [Google Scholar]
- 39.Sordi MB, Magini RS, Panahipour L, Gruber R. Pyroptosis-mediated periodontal disease. Int J Mol Sci. 2021;23:372. doi: 10.3390/ijms23010372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X, Zhang T, Xia X, Yin Y, Yang S, Ai D, Qin H, Zhou M, Song J. Pyroptosis in periodontitis: From the intricate interaction with apoptosis, NETosis, and necroptosis to the therapeutic prospects. Front Cell Infect Microbiol. 2022;12:953277. doi: 10.3389/fcimb.2022.953277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long JX, Tian MZ, Chen XY, Yu HH, Ding H, Liu F, Du K. The role of NLRP3 inflammasome-mediated pyroptosis in ischemic stroke and the intervention of traditional Chinese medicine. Front Pharmacol. 2023;14:1151196. doi: 10.3389/fphar.2023.1151196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai B, Yang Y, Wang Q, Li M, Tian C, Liu Y, Aung LHH, Li PF, Yu T, Chu XM. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020;11:776. doi: 10.1038/s41419-020-02985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, Zhang H, Qi W, Zhang Y, Li J, Li Z, Lin Y, Bai X, Liu X, Chen X, et al. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 2018;9:171. doi: 10.1038/s41419-017-0257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu Y, Li L, Guo X, Liu J, Xu L, Li Y. Exogenous spermine inhibits high glucose/oxidized LDL-induced oxidative stress and macrophage pyroptosis by activating the Nrf2 pathway. Exp Ther Med. 2022;23:310. doi: 10.3892/etm.2022.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiang SK, Chen SE, Chang LC. The role of HO-1 and its crosstalk with oxidative stress in cancer cell survival. Cells. 2021;10:2401. doi: 10.3390/cells10092401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang C, Zhang C, Yang P, Chao R, Yue Z, Li C, Guo J, Li M. Eldecalcitol inhibits LPS-induced NLRP3 inflammasome-dependent pyroptosis in human gingival fibroblasts by activating the Nrf2/HO-1 signaling pathway. Drug Des Devel Ther. 2020;14:4901–4913. doi: 10.2147/DDDT.S269223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang JY, Xu MM, Sun Y, Ding ZX, Wei YY, Zhang DW, Wang YG, Shen JL, Wu HM, Fei GH. Melatonin attenuates LPS-induced pyroptosis in acute lung injury by inhibiting NLRP3-GSDMD pathway via activating Nrf2/HO-1 signaling axis. Int Immunopharmacol. 2022;109:108782. doi: 10.1016/j.intimp.2022.108782. [DOI] [PubMed] [Google Scholar]
- 49.Zou Y, Luo X, Feng Y, Fang S, Tian J, Yu B, Li J. Luteolin prevents THP-1 macrophage pyroptosis by suppressing ROS production via Nrf2 activation. Chem Biol Interact. 2021;345:109573. doi: 10.1016/j.cbi.2021.109573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.