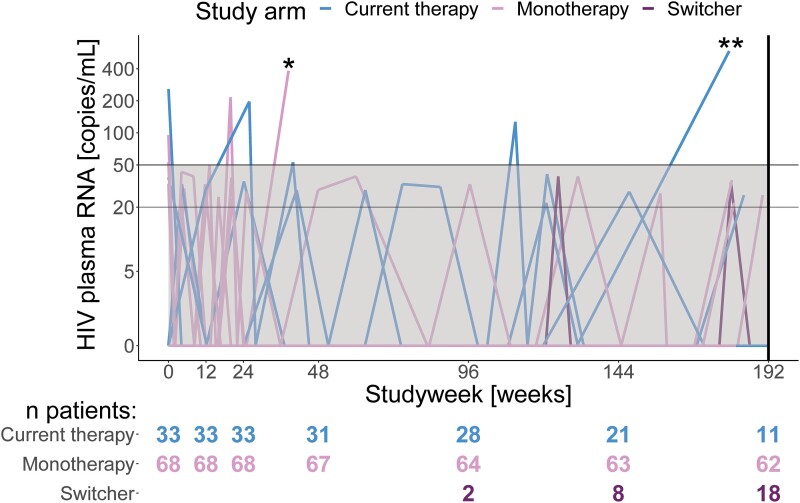
Figure 3.
HIV-1 RNA viral load over 192 weeks within HIV-1 patients receiving DTG monotherapy (n = 68) or cART (n = 33)a. Patients had the option to switch from current therapy (n = 18) to dolutegravir monotherapy, irrespective of the primary outcome (viral failure), which some patients did after week 96 or later. *One patient in the dolutegravir monotherapy group showed viral failure on dolutegravir monotherapy but was excluded from the study due to a major protocol violation. **This patient on combination anti-retroviral therapy showed a single HIV-1 plasma RNA of 586 copies/mL, which, although above the defined level of a blip, did not constitute viral failure. During the next study visit, which occurred after week 192, an undetectable viral load on the same therapy was measured. aData up to week 48 previously reported as part of the EARLY-SIMPLFIED interim analysis [12]. Abbreviations: DTG, dolutegravir; HIV-1, human immunodeficiency virus type 1.