Figure 3.
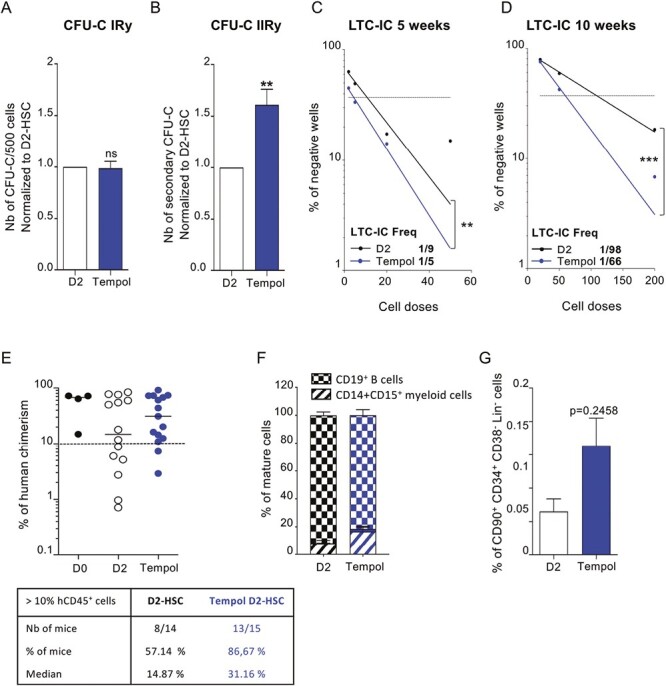
Tempol preserves long-term in vitro and in vivo HSC functionalities. (A) sorted-HSC were first pre-treated with Tempol (blue) or remained untreated (white) and then cultured for 2 days before being seeded in CFU-C medium at a density of 500 cells per plate in triplicate. Colonies (primary CFU-C) were quantified 10-12 days later. Five independent experiments. (B) Cells recovered from primary CFU-C plates were seeded in new CFU-C medium to perform serial CFU-C assay. Total cells (1%) of equivalent primary plates were seeded in triplicate. Secondary CFU-C were quantified 10-12 days later. Five independent experiments. *P < .05, **P < .01, Mann-Whitney test. (C) Representation of negative wells % from normal LTC-IC in limiting dilution (5 weeks) and quantification of LTC-IC frequency using L-CALC program (STEMCELL Technologies) between untreated D2-HSC (black, r2 = 0.911) and Tempol-treated D2-HSC (blue, r2 = 0.991). Three independent experiments (D) Representation of negative wells % from extended LTC-IC (10 weeks) in limiting dilution and quantification of LTC-IC frequency using L-CALC program (STEMCELL Technologies) between untreated D2-HSC (black, r2 = 0.997) and Tempol-treated D2-HSC (blue, r2 = 0.993). Three independent experiments. *P < .05, **P < .01, ***P < .001. (E-G) CD34+ cells were first pre-treated with Tempol (blue) or remained untreated (white) and then cultured for 2 days and grafted into NSG recipient mice. (10 000 to 25 000 CD34+ cells per mouse) 4 independent experiments. (E) % of human bone marrow chimerism obtained after 16 weeks (up) and table representing the % of mice with the % hCD45+ >10%. Each circle represents one independent mouse. (F) % of mature cells in the BM of recipient mice gated in hCD45+ fraction (Damier pattern B cells, hatched myeloid cells) (G) % of phenotypic HSC (CD34+CD38lowCD45RA−CD90+) in human cells recovered from the bone marrow.
