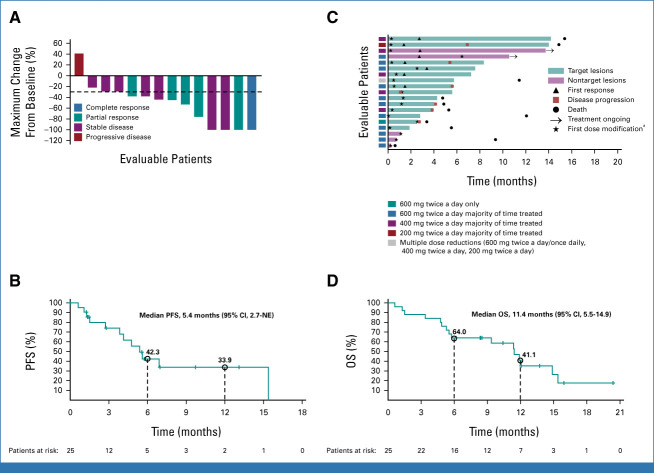
FIG 1.
Efficacy outcomes for evaluable patients per CNS RECIST v1.1 (n = 19). (A) Waterfall plot of maximum percent tumor change from baseline (only patients with target lesions are shown). (B) Kaplan-Meier graphical representation of intracranial PFS. (C) Swimmer plot showing individual duration of treatment, time to first dose modification, response, clinical outcome, and most commonly administered dose at data cutoff. (D) Kaplan-Meier graphical representation of OS for the full analysis set (N = 25). aTime to first dose modification due to any cause, including missed dose, AE, or others. For patients who had a dose modification (reduction or interruption) after initiation of treatment because of an AE, adagrasib could be restarted at the 600 mg twice a day dose following resolution of AEs if deemed appropriate. AE, adverse event; NE, not evaluable; OS, overall survival; PFS, progression-free survival.