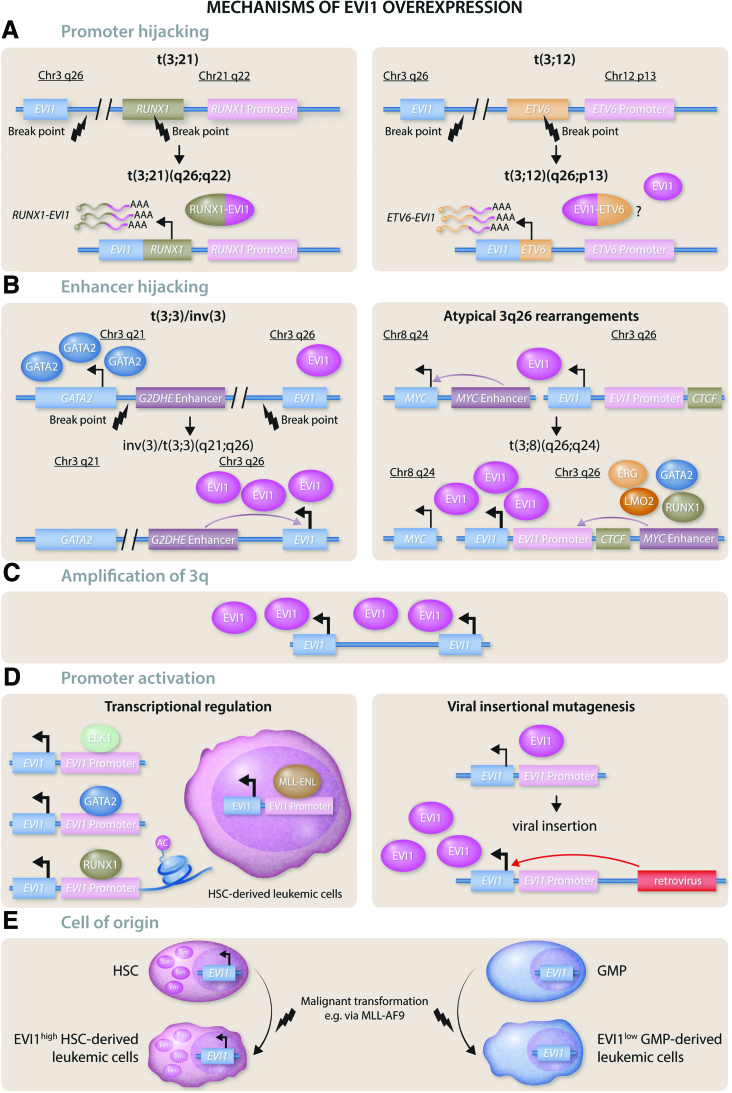
Figure 3.
Mechanisms of EVI1 overexpression. (A) Promoter hijacking: EVI1 can be aberrantly expressed through the translocation of other genes’ promoters to the MECOM locus. Left panel: In t(3;21)(q26;q22), parts of the RUNX1 gene and the RUNX1 promoter are dislocated to the MECOM locus, resulting in a RUNX1-EVI1 fusion that also encodes a RUNX1-EVI1 fusion protein. Right panel: In t(3;12)(q26;p13), parts of the ETV6 gene and the ETV6 promoter are dislocated to the MECOM locus, resulting in an ETV6-EVI1 fusion. Whether this fusion also leads to the expression of an ETV6-EVI1 fusion protein is still subject of current research. (B) Enhancer hijacking: A recurrent mechanism of EVI1 overexpression is through translocation of a potent cellular enhancer to the MECOM locus. Left panel: In t(3;3)(q21;q26) and inv(3)(q21;q26), the GATA2 distal hematopoietic enhancer (G2DHE) is dislocated to the MECOM locus, resulting in increased EVI1 levels and decreased levels of GATA2, while the expression of the MDS1-EVI1 isoform is disrupted. Right panel: The translocation t(3;8)(q26;q24 is an atypical 3q26 rearrangement resulting in the relocation of a MYC “super-enhancer” to the MECOM locus, serving as a binding site for ERG, GATA2, LMO2 and RUNX1. Upstream of the EVI1 promoter there is a CTCF enhancer-docking site that mediates promoter-enhancer looping. (C) Amplification of 3q: Amplification of 3q26q29 is a frequent event in myeloid malignancies of Fanconi anemia (FA) patients. (D) Promoter activation: Left panel: Transcriptional activation of the EVI1 locus is mediated by other transcription factors including ELK1, GATA2, and RUNX1, that bind the EVI1 promoter directly. RUNX1 also mediates acetylation of histone H3 in the EVI1 promoter region. In cells with a translocation t(11;19) (q23;p13.3), the MLL-ENL oncoprotein activates EVI1 transcription in HSC-derived leukemic cells. Right panel: The MECOM locus is a recurrent site of retroviral insertion and viral insertional mutagenesis can result in increased EVI1 expression through insertion of viral regulatory elements that aberrantly activate the EVI1 promoter. (E) Cell of origin: The epigenetic state of the cell of origin can be preserved after malignant transformation. Normal HSCs physiologically express high levels of EVI1, and leukemias arising from HSCs, for example after transformation with the MLL-AF9 oncogene, retain these high levels of EVI1. While MLL-AF9 is capable of transforming more mature myeloid progenitor cells, such progenitor-derived leukemias retain the low EVI1 expression levels present in the cell of origin.