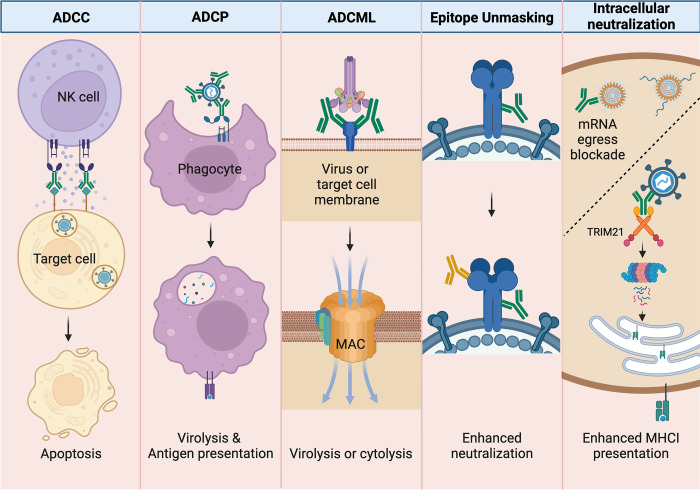
Fig 1. nNAb-mediated antiviral effector functions following antigen binding to Fab.
NK cells can exhibit ADCC by detecting target cells (i.e., virus-infected cells) opsonized by antibodies via the FcγRIII (CD16) receptor and induce apoptosis by releasing cytotoxic granules. Macrophages and other phagocytes perform ADCP by recognizing opsonized viral particles via the FcγRI (CD64) and FcγRIIA (CD32) receptors leading to virolysis and downstream antigen presentation of viral antigen. Antibodies activate the classical complement pathway after binding to the soluble complement complex, C1q. In addition to viral aggregation and opsonization, antibody-dependent complement fixation on viral or target cell membranes can lead to the formation of the pore-forming MAC and ADCML. Cooperation between nNAb (green) binding that exposes epitopes for neutralizing antibody (yellow) binding can enhance the efficacy of virus neutralization. Antibodies can block viral replication intracellularly; for dsRNA viruses that maintain an intact innermost capsid inside cells, antibodies can block mRNA egress. Intracellular antibodies can also be bound by TRIM21, which leads to proteasomal degradation of the virus–antibody complex and can result in enhanced MHC class I antigen presentation. Created with Biorender.com. ADCC, antibody-dependent cellular cytotoxicity; ADCML, antibody-dependent complement-mediated lysis; ADCP, antibody-dependent cellular phagocytosis; MAC, membrane attack complex; NK, natural killer; nNAb, nonneutralizing antibody.