Abstract
2′-Fluoro-5-methyl-β-l-arabinofuranosyluracil (l-FMAU) is the first l-nucleoside analog with low cytotoxicity discovered to have potent antiviral activities against both hepatitis B virus and Epstein-Barr virus but not human immunodeficiency virus. This spectrum of activity is different from those of the other l-nucleoside analogs examined. l-FMAU enters cells through equilibrative-sensitive and -insensitive nucleoside transport as well as through nonfacilitated passive diffusion. l-FMAU is phosphorylated stepwise in cells to its mono-, di-, and triphosphate forms. In the present study the enzymes responsible for the first step of l-FMAU phosphorylation were identified. This is the first thymidine analog shown to be a substrate not only for cytosolic thymidine kinase and mitochondrial deoxypyrimidine kinase but also for deoxycytidine kinase. This finding suggests that the antiviral activity of l-FMAU will not be limited by the loss or alteration of any of these deoxynucleoside kinases.
Hepatitis B virus (HBV) infection is a major worldwide health problem. Infection with HBV not only causes hepatitis but also has a strong association with hepatocellular carcinoma (1, 24). A selective antiviral compound would be useful for the treatment of hepatitis caused by HBV and may even prevent or delay the onset of hepatocellular carcinoma associated with HBV.
Currently, the only drug approved for the treatment of HBV hepatitis is alpha interferon. However, only 25 to 50% of patients respond to this therapy. Furthermore, there are side effects associated with this treatment (19). A more selective anti-HBV drug is needed. HBV is an incomplete double-stranded DNA virus. Its DNA replication process is quite unique and includes the step of reverse transcription catalyzed by HBV-specified DNA polymerase (23). The fact that HBV DNA polymerase is quite different from human DNA polymerase raises the possibility of the discovery of compounds that could selectively inhibit HBV DNA replication.
Deoxynucleoside analogs were previously shown to be active against HBV and to have different degrees of toxicity (22). Their mechanism of antiviral action is suggested to be mediated through a unique interaction of the triphosphate metabolites with HBV DNA polymerase. Although HBV DNA polymerase is essential for HBV DNA replication and virus propagation, it is not required for HBV supercoiled DNA or intergrated DNA synthesis. In order to be more effective in the treatment of chronic HBV infection, treatment with those anti-HBV nucleoside analogs which target HBV DNA polymerase will need to be long term. This prolonged treatment not only will inhibit HBV replication but also will deplete cells that harbor either supercoiled or integrated HBV DNA through natural turnover of virus-harboring cells. Thus, the safety of the drug upon long-term use is an important issue.
Several of the toxicities of nucleoside analogs can be associated with the incorporation of their triphosphate metabolites into nuclear DNA. Upon long-term treatment, these drugs can also interfere with mitochondrial DNA synthesis, resulting in delayed toxicity. In the search for new antiviral compounds with fewer short- and long-term toxicities, β-l-2′,3′-dideoxy-3′-thiacytidine (l-SddC; 3TC) was found to have potent activity against HBV and human immunodeficiency virus (HIV) (3, 9). Nucleosides are found in nature only in the β-d configuration, and this compound was the first nucleoside analog with the unnatural β-l configuration shown to have biological activity. Subsequently, several other β-l-deoxycytidine analogs were found to have similar activities against HBV and HIV (10, 11, 16, 17, 29). However, none of these compounds is active against Epstein-Barr virus (EBV).
l-SddC exerts its antiviral activity through the interaction of its triphosphate metabolite with HIV reverse transcriptase and HBV DNA polymerase. Unlike most previously studied antiviral dideoxynucleoside analogs including the anti-HBV compounds 2′-fluoro-5-methyl-β-d-arabinofuranosyluracil (d-FMAU) and 1-(2′-deoxy-2′-fluoro-β-d-arabinofuranosyl)-5-iodouracil (d-FIAU), l-SddC does not interfere with mitochondrial function. l-SddC has already been approved for use for the treatment of patients with AIDS in combination with zidovudine. In addition, l-SddC is currently showing impressive results in clinical trials for the treatment of chronic HBV infection (18, 25).
A problem with the use of l-SddC for anti-HIV therapy is the development of resistance mediated by mutations in the HIV reverse transcriptase. Although l-SddC-resistant viruses are likely to be cross-resistant to other deoxycytidine (dCyd) analogs, they may not be resistant to analogs with different base. In addition, the spectrums of toxicity and activity of these analogs might be different from those of analogs with a cytidine base. Therefore, several l-nucleoside analogs with different bases were synthesized and examined for antiviral activity. One of these compounds, 2′-fluoro-5-methyl-β-l-arabinofuranosyluracil (l-FMAU), was found to be active against HBV in 2.2.15 cells, with a 50% effective concentration of 0.1 μM. Unlike the β-l-dideoxycytidine (β-l-ddC) analogs, this compound was inactive against HIV. Interestingly, it was also active against EBV, with a 90% effective concentration of 5 μM (7, 21). This is the first example of an l-thymidine analog with potent antiviral activity.
Although its spectrum of activity is different from those of other β-l-nucleoside analogs, it shares their favorable toxicity profiles. Unlike its d analog, l-FMAU triphosphate (l-FMAUTP) could not be incorporated into nuclear DNA on the basis of cell culture studies and experiments done with purified human DNA polymerases (21). In addition, treatment with l-FMAU did not deplete cells of mitochondrial DNA. Therefore, delayed toxicities upon long-term treatments should not be a problem with this compound. Indeed, no toxicity was observed in mice after 30 days of continuous treatment with l-FMAU at 50 mg/kg of body weight. In vivo, l-FMAU was shown to have potent antiviral activity against duck HBV when it was administered at an oral dosage of 10 mg/kg for 5 days (30). These results demonstrate the potential use of l-FMAU for the treatment of human HBV infection.
Like other nucleoside analogs, l-FMAU could be phosphorylated in cells to its mono-, di-, and triphosphate metabolites. l-FMAUTP is the major metabolite in most cell lines examined except H1 cells, in which l-FMAU monophosphate (l-FMAUMP) is the major metabolite (27, 28). This unique metabolic feature of l-FMAU in H1 cells could be due to the presence of EBV thymidine (dThd) kinase in cells. The metabolism of l-FMAU in 2.2.15 cells was no different from its metabolism in HepG2 cells, suggesting that the activation of l-FMAU is most likely carried out by cellular enzymes (21). Given the unique structure of l-FMAU, it was not clear which enzymes in human cells could be important in its metabolism. This report describes the results of our studies with respect to the enzymes with key roles in the phosphorylation of l-FMAU to l-FMAUTP.
MATERIALS AND METHODS
Chemicals.
l-FMAU was synthesized by C. K. Chu, Department of Chemistry, University of Georgia. [methyl-3H(N)]l-FMAU (76 Ci/mmol), [5′-3H]l-FMAU (29.8 Ci/mmol), [methyl-14C]thymidine (54 mCi/mmol), [2-14C]2′-deoxycytidine (56 mCi/mmol), and [2′,3′-3H]dideoxyinosine (42 Ci/mmol) were purchased from Moravek Biochemicals, Inc., Brea, Calif. Both l-FMAU and [3H]l-FMAU were further purified by high-performance liquid chromatography (HPLC) on an RP-C18 column (Alltech Associates, Inc., Deerfield, Ill.) and eluted with 20 mM KH2PO4 (pH 5.5). l-FMAUTP was synthesized by a previously described procedure (3). 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), streptomycin sulfate, dipyridamole (DPM), and b-[(4-nitrobenzyl)thio]-9-β-d-ribofuranosyl purine (NBMPR) were purchased from Sigma Chemical Co., St. Louis, Mo.
Purification of cytosolic dThd kinase and mitochondrial dPyd kinase.
The purification procedures were the same as those described previously, with some modifications (15). Human chronic lymphocytic leukemia cells were extracted with 3 volumes of buffer containing 10 mM Tris-HCl (pH 7.5), 1.5 mM MgCl2, 3 mM dithiothreitol (DTT), and 10 μM dThd. After 0.5% streptomycin sulfate precipitation and 55% ammonium sulfate precipitation, followed by dialysis, the crude cell extract was purified with a dThd affinity column (13) (0.5 by 5 cm). 5-Bromovinyl-deoxyuridine inhibited mitochondrial deoxypyrimidine (dPyd) kinase activity (Ki = 0.83 ± 0.12 μM) but had no effect on that of cytosolic dThd kinase (Ki > 100 μM) (5). CHAPS (0.5 mM) was added to each fraction to stabilize the enzymes (20). Due to the low protein concentration of purified enzyme, the specific activity of cytosolic dThd kinase was estimated to be higher than 37 U/mg, while that of mitochondrial dPyd kinase was higher than 6 U/mg. The unit of specific activity is defined as the amount of enzyme which converts 1 nmol of dThd in 1 min at 37°C under our assay conditions.
Purification of dCyd kinase.
dCyd kinase was purified from BL21 (de3) bacteria containing the PET-3d expression vector into which the cDNA of the human dCK gene cloned from KB cells has been inserted. The bacterial pellet was extracted with buffer containing 25 mM Tris-HCl (pH 7.5), 2 mM DTT, 10% glycerol, and 40 μM ATP-MgCl2 and was lysed by treatment with 10 μg of lysozyme per ml and sonicated. After centrifugation to remove the insoluble fraction, the supernatant was fractionated with 20 to 55% ammonium sulfate. The ammonium sulfate was then removed by dialysis against buffer containing 25 mM Tris-HCl (pH 7.5), 2 mM DTT, 10% glycerol, and 40 μM ATP-MgCl2. The protein was then loaded onto a DE-52 anion-exchange column and eluted with a linear gradient of the same buffer containing 0 to 1 M NaCl (4). The partially purified protein was then loaded onto a hydroxyapatite column and was eluted with a linear gradient of 0 to 1.0 mM ATP-MgCl2. The dCyd kinase was further purified by fast performance liquid chromatography with an anion-exchange mono-Q column and had a final specific activity of 13 U/mg. The unit of specific activity is defined as the amount of enzyme which converts 1 nmol of dCyd in 1 min at 37°C. Bovine serum albumin (1 mg/ml) was added to stabilize the purified dCyd kinase. No contamination of dThd kinase was found in the dCyd kinase preparation.
dThd kinase and dCyd kinase activity assay.
dThd kinase (0.004 U for cytosolic dThd kinase or 0.0006 U for mitochondrial dThd kinase) or dCyd kinase (0.01 U) was incubated at 37°C for 1 h with the kinase mixture which was described previously (4, 26), but with the following modifications: 0.14 M Tris-HCl (pH 7.5), 1.7 mM DTT, 8 mM NaF, 2 mM ATP-MgCl2, and 0.1 mM [14C]dThd (11.8 mCi/mmol) or 0.1 mM [14C]dCyd (11.8 mCi/mmol). The same procedures were used for the kinetic studies, with the exception that the reaction mixtures contained different concentrations of dThd, ranging from 2 to 20 μM, or dCyd, ranging from 1.25 to 40 μM (with radiospecificities of 19.9 mCi/mmol for both substrates).
l-FMAU kinase activity assay.
The reaction conditions of the l-FMAU kinase activity assay were similar to those of dThd kinase assays except that 0.1 mM [3H]l-FMAU (1,000 μCi/μmol) was used as a substrate. Sometimes, UTP was used to replace ATP for dCyd kinase catalysis. After the reaction, 50 μl of each reaction mixture was applied to a Whatman DE-81 disc, and then the disc was washed three times with 1 mM ammonium formate and once with ethanol. The discs were dried and 1 ml of 0.2 N HCl–2 M NaCl was added to elute the radioactive product from the disc. Radioactivities were quantified by scintillation counting. For the kinetic studies with dThd kinase, l-FMAU concentrations ranging from 5 to 40 μM were used. For the kinetic studies with dCyd kinase, the concentration of l-FMAU ranged from 50 to 800 μM. In examining the inhibitory effect of natural nucleosides, 588 μM nucleoside was used with 100 μM [3H]l-FMAU as a substrate.
5′-Nucleotidase activity assay.
The 5′-nucleotidase activity assay proctols were modified from the procedures reported by Johnson and Fridland (12). The 5′-nucleotidase reaction mixture contained 0.1 M Tris-HCl (pH 7.5), 5 mM MgCl2, 3 mM DTT, 0.5 M KCl, 10 μM dideoxyinosine (ddI), 1 μCi of [3H]ddI, 2 mM IMP, and dialyzed cell extract. The reaction mixture was incubated at 37°C for 2 h in a final volume of 100 μl. The resulting products were detected by the procedures described above.
Drug metabolism analysis.
The cells (3 × 107) were incubated with 1 μM [3H]l-FMAU (14.5 μCi/mmol) at 37°C for specific periods of the time in the presence or absence of dCyd (20 μM) and/or dThd (20 μM) as a competitor. The metabolites were extracted with perchloric acid and analyzed by HPLC by previously described procedures (27). The intracellular nucleotide concentration was normalized with the cell number and an internal standard, acyclovir triphosphate.
Drug transport.
The inhibitor stop method was used in transport studies (8, 14). A total of 4 × 105 2.2.15, HepG2, HeLa, or DU-145 cells were seeded per 35-mm dish, and the dishes were incubated for 48 h. The cells were incubated with either NBMPR (0.3 nM to 20 μM), DPM (30 nM to 20 μM), or adenine (0.5 mM) at 37°C for 15 min prior to the transport assays. [14C]dThd (53 mCi/mmol) was added to the cells for times ranging from 2 to 30 s, and 5′-[3H]l-FMAU (200 mCi/mmol) was added for times ranging from 2 s to 30 min. Transport was terminated by the addition of ice-cold phosphate-buffered saline containing 20 μM DPM and placing the dish on ice. The cells were then washed five times with cold phosphate-buffered saline containing 20 μM DPM and were solubilized with 1% Sarkosyl. The radioactivities were determined in a liquid scintillation counter.
RESULTS
Inhibition of l-FMAU phosphorylation by natural nucleosides.
In order to identify which nucleoside kinase could potentially be responsible for the first step of phosphorylation of l-FMAU, the effects of different natural nucleosides on [3H]l-FMAU phosphorylation catalyzed by a dialyzed crude HepG2 cell extract were examined. The fact that these natural nucleosides could compete with [3H]l-FMAU for phosphorylation indicated that a unique kinase was used. As indicated in Fig. 1, dThd, a substrate of cytosolic dThd kinase and mitochondrial dPyd kinase, inhibited about 90% of l-FMAU phosphorylation, while dCyd, a substrate of cytosolic dCyd kinase and mitochondrial dPyd kinase, inhibited 60% of l-FMAU phosphorylation. These data suggest that cytosolic dThd kinase, cytosolic dCyd kinase, and mitochondrial dPyd kinase all may play a role in the phosphorylation of l-FMAU. Although deoxyadenosine (dAdo) and deoxyguanosine (dGuo) are substrates of cytosolic dCyd kinase, l-FMAU phosphorylation was not affected significantly by dAdo or dGuo. This observation could be due to the poor binding affinity of dAdo and dGuo for cytosolic dCyd kinase, the lack of action of dAdo and dGuo against mitochondrial dPyd kinase, or the higher rate of catabolism of dAdo and dGuo by adenosine deaminase and purine nucleoside phosphorylase, respectively, in the crude HepG2 cell extract. Furthermore, the major enzyme for l-FMAU phosphorylation in this crude extract is likely to be cytosolic dThd kinase since dThd can inhibit most l-FMAU phosphorylation.
FIG. 1.
Effects of nucleosides on l-FMAU phosphorylation by HepG2 cell extracts. [3H]l-FMAU (100 μM) was phosphorylated with dialyzed HepG2 cell extracts in the presence or absence of nucleosides at 588 μM. The values are means ± standard deviations of three determinations. Ado, adenosine; Cyd, cytidine; Guo, guanosine; Urd, uridine; dAdo, deoxyadenosine; dGuo, deoxyguanosine; dUrd, deoxyuridine.
Behavior of l-FMAU toward cytosolic dThd kinase and cytosolic dCyd kinase.
Both cytosolic dThd kinase and cytosolic dCyd kinase were prepared as described above. The behavior of l-FMAU as a substrate for those two enzymes was examined. The results are depicted in Table 1. The Km value of l-FMAU for cytosolic dThd kinase was 18.3 μM, which is threefold greater than that of dThd (Km = 6 μM). However, the Vmax of l-FMAU was similar to that of dThd. Therefore, the relative ratio of Vmax/Km, which is often used to indicate enzyme efficiency when the concentration of the substrate used is relatively low in comparison with the Km, for l-FMAU phosphorylation by cytosolic dThd kinase was one-third that for dThd. To ensure that the reaction studied was carried out by cytosolic dThd kinase and not by some contaminating activity, the effects of dThd, a substrate, and dTTP, an inhibitor of cytosolic dThd kinase, were examined. dThd was found to be a competitive inhibitor of l-FMAU phosphorylation, with a Ki of 7 μM, while TTP served as an inhibitor of l-FMAU phosphorylation, with 50% inhibition occurring with l-FMAU at 4 μM (Table 2).
TABLE 1.
Kinetic constants of l-FMAU phosphorylation by cellular kinasesa
| Compound | Cytosolic dCyd kinase
|
Cytosolic dThd kinase
|
Mitochondrial dPyd kinase
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Km (μM) | Vmax | Vmax/Km | Km (μM) | Vmax | Vmax/Km | Km (μM) | Vmax | Vmax/Km | |
| dCyd | 7 ± 0 | 1 | 0.14 | NDb | ND | ND | 33 ± 2 | 1.4 | 0.04 |
| dThd | ND | ND | ND | 6 | 1 | 0.17 | 7 ± 0.2 | 1 | 0.14 |
| L-FMAU | |||||||||
| ATPc | 1,125 ± 90 | 12.5 | 0.01 | 18.3 ± 1.4 | 1 | 0.05 | 61 ± 5 | 2.5 | 0.04 |
| UTPc | 250 ± 60 | 8.0 | 0.03 | ||||||
The Km and relative Vmax values were determined by a Lineweaver-Burk plot. The values are the means ± standard deviations of three determinations. Vmax valves are those relative to the Vmax for the substrate.
ND, not detectable.
Phosphate donor.
TABLE 2.
Substrate inhibition and feedback inhibition of l-FMAU phosphorylation by cellular kinasesa
| Enzyme |
Ki (μM)
|
IC50 (μM)b
|
||
|---|---|---|---|---|
| dThd | dCyd | TTP | dCTP | |
| Cytosolic dThd kinase | 7 ± 0.1 | NDc | 4 ± 1 | ND |
| Cytosolic dCyd kinase | ND | 1.2 ± 0.1 | ND | 0.8 ± 0.5 |
| Mitochondrial dPyd kinase | 10.8 ± 2 | >100 | 12 ± 2 | 6 ± 0.1 |
The values presented here are the averages of triplicate experiments. Ki was determined by using the equation for a competitive inhibitor.
IC50, concentration required for 50% inhibition of l-FMAU phosphorylation.
ND, not detectable.
The discovery that dCyd could inhibit l-FMAU phosphorylation in crude cell extracts suggested that dCyd kinase might also be able to phosphorylate l-FMAU. The behavior of l-FMAU as a substrate for cytosolic dCyd kinase was studied. l-FMAU was phosphorylated by cytosolic dCyd kinase with a Km of 1.1 mM, which is much higher than that of dCyd in the presence of ATP. However, the Vmax of l-FMAU was 12.5-fold higher than that of dCyd. The Vmax/Km of l-FMAU as a substrate for dCyd kinase was 14-fold lower than that of dCyd in the presence of ATP (Table 1). UTP has been shown to be a good phosphate donor of cytosolic dCyd kinase. The Km of l-FMAU was determined to be 0.25 mM, and the Vmax was determined to be 8-fold that of dCyd when UTP was the phosphate donor. The Vmax/Km for l-FMAU phosphorylation with UTP as a phosphate donor was 4.7-fold lower than that for dCyd. In addition, the effects of dCyd and dCTP as inhibitors of l-FMAU phosphorylation catalyzed by this enzyme were examined. dCyd was found to be an effective competitive inhibitor of l-FMAU phosphorylation, with a Ki value of 1.2 μM, while dCTP showed 50% inhibition at 0.8 μM, as indicated in Table 2.
Behavior of l-FMAU toward mitochondrial dPyd kinase.
l-FMAU was also found to be a substrate for mitochondrial dPyd kinase, with a Km value higher than that of either dCyd or dThd (Km values, 61, 33, and 7 μM, respectively). The relative Vmax of l-FMAU was also higher than that of dCyd or dThd, as indicated in Table 1. Therefore, the Vmax/Km for l-FMAU phosphorylation by mitochondrial dPyd kinase was about the same as that for dCyd and lower than that for dThd. dThd was also shown to be a competitive inhibitor of l-FMAU phosphorylation catalyzed by mitochondrial dPyd kinase, with a Ki of 10.8 μM, while dCyd was a poor inhibitor, with a Ki of >100 μM. Both TTP and dCTP proved to be potent inhibitors of l-FMAU phosphorylation, with 50% inhibitory concentrations of 12 and 6 μM, respectively (Table 2).
Behavior of l-FMAU toward 5′-nucleotidase.
Since cytosolic 5′-nucleotidase was shown to be responsible for the intracellular phosphorylation of ddI, the ability of this enzyme to phosphorylate l-FMAU was examined. Under the same reaction conditions, ddI was phosphorylated to ddIMP in the absence of ATP, while no trace of l-FMAUMP formation was observed. Therefore, it is unlikely that 5′-nucleotidase is able to phosphorylate l-FMAU.
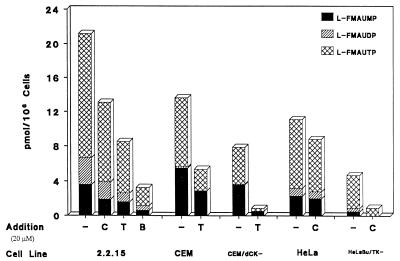
Inhibition of phosphorylation of l-FMAU by dThd and dCyd in 2.2.15 cells.
The HBV-producing 2.2.15 cells were incubated with 1 μM [3H]l-FMAU with and without dCyd or dThd for 4 h. The metabolism of l-FMAU was examined. With increasing dThd or dCyd concentrations, the intracellular l-FMAU nucleotide concentrations dropped sequentially. When a 20-fold excess of dCyd was added, the amount of l-FMAU nucleotides dropped to ∼60% of that of the control. With a 20-fold excess of dThd present, the intracellular l-FMAU nucleotide levels decreased to 40% of that of the control, as indicated in Fig. 2. When both dThd and dCyd were administered with [3H]l-FMAU, the intracellular l-FMAU nucleotide concentration decreased to 15% of that of the control. The degree of inhibition with the combination of dCyd and dThd is lower than that which is expected on the basis of the enzyme studies. This could be due to some difference in enzyme behavior in cells from that in the isolated form of the enzyme. These data are consistent with the observation that both dCyd kinase and dThd kinase could phosphorylate l-FMAU.
FIG. 2.
l-FMAU metabolism in the presence of dThd, dCyd, or both. Cells (3 × 107) were incubated with 1 μM [3H]l-FMAU (14.5 μCi/mmol) at 37°C for 4 h in the absence or presence of 20 μM dThd, dCyd, or both. The intracellular nucleotide concentrations were analyzed by HPLC as described in Materials and Methods. CEM/dCK−, cytosolic dCyd kinase-deficient CEM cell line; HeLa Bu/TK−, cytosolic dThyd kinase-deficient HeLa cells; −, control; C, deoxycytidine; T, thymidine; B, both.
Phosphorylation of l-FMAU in cytosolic CEM/dCK− cells.
To ensure the role of dCyd kinase in the phosphorylation of l-FMAU, CEM cells and CEM cytosolic dCyd kinase-deficient (CEM/dCK−) cells were compared with respect to l-FMAU metabolism (Fig. 2). There was a decrease in the amount of phosphorylated metabolites in CEM/dCK− cells in comparison with that in CEM cells. The phosphorylation of l-FMAU in CEM/dCK− cells could be due to cytosolic dThd kinase and mitochondrial dPyd kinase in the cells. The addition of dThd had a more pronounced effect on the inhibition of l-FMAU phosphorylation in CEM/dCK− cells.
Phosphorylation of l-FMAU in HeLa Bu cells.
The metabolism of l-FMAU in the HeLa and the HeLa cytosolic dThd kinase-deficient (HeLa Bu) cell lines was examined. The addition of dCyd had a more pronounced effect on the inhibition of l-FMAU phosphorylation in HeLa Bu cells than in HeLa cells, as indicated in Fig. 2. The phosphorylation of l-FMAU in HeLa Bu cells could be due to the presence of cytosolic dCyd kinase and mitochondrial dPyd kinase.
Transport behaviors of l-FMAU.
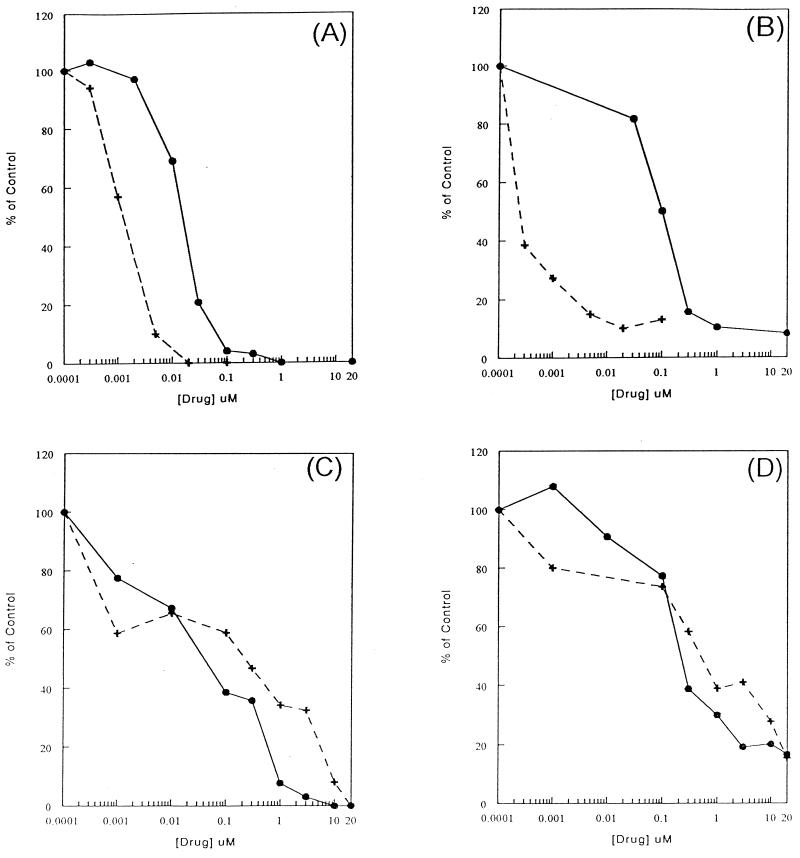
Since the inhibition of l-FMAU phosphorylation by dThd or dCyd in cells could partly be due to the competition with dThd and dCyd for l-FMAU transport into cells, the transport of l-FMAU into HepG2 cells was examined in the presence of the nucleoside-facilitated diffusion inhibitors NBMPR and DPM. [14C]dThd (56 mCi/mmol) was used to characterize the nucleoside transport system in HepG2 cells. dThd transport was inhibited by both DPM and NBMPR, with 50% inhibition occurring at 1 and 20 nM, respectively, in a 30-s reaction (Fig. 3A). The transport of dThd was completely inhibited when DPM or NBMPR concentrations were higher than 1 μM. This observation suggested that the equilibrative-sensitive system was the only nucleoside transport system present in HepG2 cells. The transport of l-FMAU was much slower than that of dThd. The rate of l-FMAU uptake is 60-fold less than that of dThd uptake. l-FMAU uptake was sensitive to inhibition by NBMPR, with 50% inhibition at 0.1 nM, while higher concentrations of DPM (0.1 μM) were required to achieve the same degree of inhibition (Fig. 3B). These data indicate that the equilibrative-sensitive nucleoside transport system could also be used for l-FMAU uptake by HepG2 cells. It was noted that neither inhibitor could inhibit l-FMAU uptake 100%. Ten percent of l-FMAU was still transported into HepG2 cells in the presence of 20 μM DPM or NBMPR, while no dThd was found. When the l-FMAU uptake study was performed at 4°C, similar amount of uptake occurred. These results suggest that a passive diffusion mechanism could also play a role in l-FMAU uptake. Since HepG2 cells used only the equilibrative-sensitive nucleoside transport system, other transport systems were examined in other cell lines.
FIG. 3.
Characterization of human cellular l-FMAU uptake system. (A) HepG2 cells were preincubated with either DPM (•) or NBMPR (+) before the transport assay. Then, 3 μM [14C]dThd (53 mCi/mmol) was added to the cells for 30 s and the transport was terminated as described in Materials and Methods. (B) [3H]l-FMAU (10 μM; 200 mCi/mmol) was transported into DPM- or NBMPR-treated HepG2 cells for 30 min. (C) Transport of 3 μM [14C]dThd into HeLa cells. (D) Transport of 10 μM [3H]l-FMAU into HeLa cells.
HeLa cells were reported to have two classes of nucleoside transport systems, the equilibrative-sensitive system with high affinity to NBMPR and the equalibritive-insensitive system with low affinity to NBMPR (8, 14). By using dThd as a control, the dThd transport was inhibited 40% at an NBMPR concentration of 1 nM. The remaining 60% of the dThd uptake remained insensitive to NBMPR until the concentration of NBMPR was elevated 1,000-fold (Fig. 3C). This suggests that the transport of dThd is approximately 40% through the equilibrative-sensitive system and 60% through the equilibrative-insensitive system. The inhibition of l-FMAU uptake by NBMPR and DPM is similar to that of dThd (Fig. 3D). These observations suggest that l-FMAU could also be transported into HeLa cells through the equilibrative-insensitive system.
The nucleoside analog carbovir was shown to be a substrate for the nucleobase carrier, which is inhibited by adenine. Therefore, the ability of adenine to inhibit l-FMAU transport was also examined in DU-145 cells. [3H]fluorouracil ([3H]FU; 100 mCi/mmol) was used as a control for nucleobase transport. The transport of FU was inhibited in the presence of adenine. When the concentration of adenine was as high as 0.5 mM, 85% of FU transport was inhibited, while no inhibition of l-FMAU transport was found. These data suggested that l-FMAU is not a substrate for the nucleobase transporter.
DISCUSSION
l-FMAU is the first thymidine analog shown to have potent anti-HBV and anti-EBV activity in cell culture. This spectrum of activity is different from those of other biologically active l-dideoxythymidine analogs (2).
l-FMAUTP acts as a potent inhibitor of HBV DNA polymerase with a Ki of 0.12 μM (calculated from previously published data [21]). It cannot be used as a substrate by human α, β, γ, and δ DNA polymerases. This may explain its selective antiviral activity against HBV and its lack of toxicity. In this report the enzymes responsible for the first step in the phosphorylation of l-FMAU were identified. Unlike other dThd or dCyd analogs, l-FMAU could be phosphorylated by cytosolic dThd kinase, cytosolic dCyd kinase, and mitochondrial dPyrd kinase as a substrate. The phosphorylation of l-FMAU by those enzymes was also subject to inhibition by their feedback inhibitors. When the efficiency (Vmax/Km) of l-FMAU as a substrate for all three enzymes was examined, it was found to be within the same order of magnitude as those of the natural substrates. Chou et al. (6) reported that d-FMAU is a competitive inhibitor for the incorporation of dThd and dCyd into DNA in mouse leukemia p815 cells and dThd in cytosine arabinoside (Ara-C)-resistant p815/Ara-C cells. Although this could suggest that d-FMAU is a substrate for both dThd kinase and dCyd kinase, several other mechanisms could be responsible for the observation, such as the competition of d-FMAUTP with dTTP or dCTP at the DNA polymerase level. It would be interesting to determine whether d-FMAU could also serve as a substrate for both cytosolic kinase and cytosolic dCyd kinase. Therefore, to the best of our knowledge, l-FMAU is the first pyrimidine nucleoside analog shown to be a substrate for all three cellular deoxypyrimidine nucleoside kinases.
In order to further investigate whether l-FMAU could be phosphorylated by cytosolic dCyd kinase or dThd kinase, we studied the metabolism of l-FMAU in cells that lack these enzymes. l-FMAU was phosphorylated less, but it was still phosphorylated substantially in either enzyme-deficient cell line. dThd and dCyd influenced the degree of l-FMAU phosphorylation in those cell lines. The inhibition of l-FMAU phosphorylation by dThd in cytosolic dCyd kinase-deficient cell lines than was more pronounced than that in cells with all three enzymes. This is due to the fact that cytosolic dThd kinase is the only major enzyme responsible for l-FMAU phosphorylation in these cells. Likewise, the phosphorylation of l-FMAU is much less influenced by dCyd in HeLa cells than in HeLa Bu cells. This observation is consistent with our enzyme studies in which the action of dCyd and/or dThd on l-FMAU metabolism was studied in HepG2 cells. Both dThd and dCyd could suppress l-FMAU phosphorylation to some extent. However, the combination of dThd and dCyd was the most effective.
Our concern in this cell culture study was that dThd or dCyd could inhibit the transport of l-FMAU through the plasma cell membrane, resulting in the inhibition of l-FMAU phosphorylation intracellularly if these compounds use the same transport system. There are two facilitated transport systems, equilibrative-sensitive and equilibrative-insensitive systems, for the transport of nucleoside in culture cells. HepG2 cells have predominately equilibrative-sensitive transporters, while HeLa cells have 40% equilibrative-sensitive and 60% equilibrative-insensitive transporters under the conditions used in this study. We found that l-FMAU could use both equilibrative-sensitive and equilibrative-insensitive facilitated nucleoside transport systems. In addition, l-FMAU appears to be able to enter cells via passive diffusion. Thus, the inhibition of l-FMAU phosphorylation in HepG2 cells is the combined effect of competition by dThd and/or dCyd for uptake as well as competition for phosphorylation by cellular kinases.
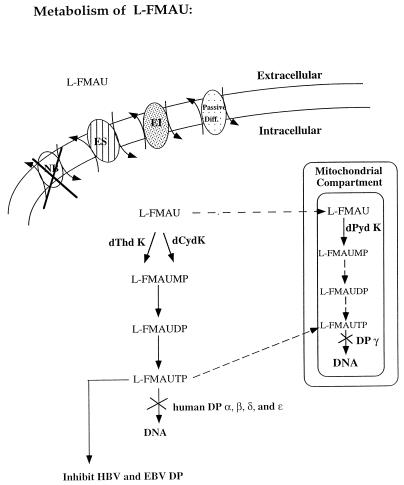
In summary, as shown in Fig. 4, l-FMAU is a unique nucleoside analog that can be recognized as a substrate by all three nucleoside kinases. In vivo, it is likely that cytosolic dCyd kinase is the major enzyme for phosphorylation in the liver since the activities of both cytosolic dThd kinase and mitochondrial dPyd kinase are low in comparison with the activity of cytosolic dCyd kinase. It is not clear whether l-FMAU could be taken into mitochondria to be phosphorylated, since this compound does not interfere with mitochondrial function like its d-FMAU enantiomer does. Thus, the ability of mitochondrial dPyd nucleoside kinase to phosphorylate l-FMAU may not have functional significance. This compound is in preclinical development for the treatment of patients with HBV infection.
FIG. 4.
Summary of l-FMAU metabolism in the cells. ES, equilibrative-sensitive nucleoside transport system; EI, equilibrative-insensitive nucleoside transport system; NB, nucleobase transport system; Passive Diff., passive diffusion system; DP, DNA polymerase. Arrows with solid line indicate the reactions that are confirmed, while arrows with dashed lines indicate the reactions that are unconfirmed.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI33655 and AI 38204.
REFERENCES
- 1.Ayoola E A, Balayan M S, Deinhardt F, Gust I, Kureshi A W, Maynaed J E, Nayak N C, Brodley D W, Ferguson M, Melnick J, Purcell R H, Zuckerman A J. Progress in the control of viral hepatitis: memorandum from a WHO meeting. Bull W H O. 1988;66:443–455. [PMC free article] [PubMed] [Google Scholar]
- 2.Bridges E G, Cheng Y-C. Use of novel β-l(−)-nucleoside analogues for treatment and prevention of chronic hepatitis B virus infection and hepatocellular carcinoma. Prog Liver Dis. 1996;13:231–245. [PubMed] [Google Scholar]
- 3.Chang C-N, Doong S-L, Zhou J H, Beach J W, Jeong L S, Chu C K, Tsai C-H, Cheng Y-C. Deoxycytidine deaminase-resistant stereoisomer is the active form of (±)-2′-3′-dideoxy-3′-thiacytidine in the inhibition of hepatitis B virus replication. J Biol Chem. 1992;267:13938–13942. [PubMed] [Google Scholar]
- 4.Cheng Y C, Domin B, Lee L-S. Human deoxycytidine kinase. Purification and characterization of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia patients. Biochim Biophys Acta. 1977;481:481–492. doi: 10.1016/0005-2744(77)90281-9. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y-C, Dutschman G, Fox J J, Watanabe K A, Machida H. Differential activity of potential antiviral nucleoside analogs on herpes simplex virus-induced and human cellular thymidine kinases. Antimicrob Agents Chemother. 1981;20:420–423. doi: 10.1128/aac.20.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou T-C, Burchenal J H, Schmid F A, Braun T J, Su T-L, Watanale K A, Fox J J, Philips F S. Biochemical effects of 2′-fluoro-5-methyl-1-β-d-arabinofuranosyluracil and 2′-fluoro-5-iodo-1-β-d-arabinofuranosylcytidine in mouse leukemic cells sensitive and resistant to 1-β-d-arabinofuranosylcytosine. Cancer Res. 1982;42:3957–3963. [PubMed] [Google Scholar]
- 7.Chu C K, Ma T, Shammuganathan K, Wang C, Xiang Y, Pai S B, Yao G Q, Sommadossi J P, Cheng Y C. Use of 2′-fluoro-5-β-l-arabinofuranosyl uracil as a novel antiviral agent for hepatitis B virus and Epstein-Barr virus. Antimicrob Agents Chemother. 1995;39:979–981. doi: 10.1128/aac.39.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahlig-Harley E, Eilam Y, Paterson A R, Cass C E. Binding of nitrobenzylthioinosine to high-affinity sites on the nucleoside-transport mechanism of HeLa cells. Biochem J. 1981;200:295–305. doi: 10.1042/bj2000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doong S-L, Tsai C-H, Schinazi R F, Liotta D C, Cheng Y-C. Inhibition of the replication of hepatitis B virus in vitro by 2′-3′-dideoxy-3′-thiacytidine and related analogues. Proc Natl Acad Sci USA. 1991;88:8495–8499. doi: 10.1073/pnas.88.19.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furman P A, Davis M, Liotta D C, Paff M, Frick L W, Nelson D J, Dornsife R E, Wurster J A, Wilson L J, Fyfe J A. The anti-hepatitis B virus activities, cytotoxicities, and anabolic profiles of the (−) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992;36:2686–2692. doi: 10.1128/aac.36.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gosselin G, Schinazi R F, Sommadossi J P, Mathe C, Bergogne M C, Aubertin A M, Kirn A, Imbach J L. Anti-human immunodeficiency virus activities of the β-l-enantiomer of 2′-3′-dideoxycytidine and its 5-fluoro derivative in vitro. Antimicrob Agents Chemother. 1994;38:1292–1297. doi: 10.1128/aac.38.6.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson M A, Fridland A. Phosphorylation of 2′-3′-dideoxyinosine by cytosolic 5′-nucleotidase of human lymphoid cells. Mol Pharmacol. 1989;36:291–295. [PubMed] [Google Scholar]
- 13.Kowal E P, Markus G. Affinity chromatography of thymidine kinase from a rat colon adenocarcinoma. Prep Biochem. 1976;6:369–385. doi: 10.1080/00327487608061625. [DOI] [PubMed] [Google Scholar]
- 14.Lauzon G J, Paterson A R P. Binding of the nucleoside transport inhibitor nitrobenzylthioinosine to HeLa cells. Mol Pharmacol. 1977;13:883–891. [PubMed] [Google Scholar]
- 15.Lee L S, Cheng Y C. Human deoxythymidine kinase. I. Purification and general properties of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia. J Biol Chem. 1976;251:2600–2604. [PubMed] [Google Scholar]
- 16.Lee M, Chu C K, Pai S B, Zhu Y-L, Cheng Y-C, Chun M W, Chung W K. Dioxolane cytosine nucleosides as anti-hepatitis B agents. Bioorg Med Chem Lett. 1995;17:2011–2014. [Google Scholar]
- 17.Lin T-S, Luo M-Z, Liu M-C, Zhu Y-L, Gullen E, Dutschman G E, Cheng Y-C. Design and synthesis of 2′,3′-dideoxy-2′,3′-didehydro-β-l-cytidine (β-l-d4C) and 2′,3′-dideoxy-2′,3′-didehydro-β-l-5-fluorocytidine (β-l-Fd4C), two exceptionally potent inhibitors of human hepatitis B virus (HBV) and potent inhibitors of human immunodeficiency virus (HIV) in vitro. J Med Chem. 1996;39:1757–1759. doi: 10.1021/jm950836q. [DOI] [PubMed] [Google Scholar]
- 18.Ling R, Mutimer D, Ahmed M, Boxall E H, Elias E, Dusheiko G M, Harrison T J. Selection of mutations in the hepatitis B virus polymerase during therapy of transplant recipients with lamivudine. Hepatology. 1996;24:711–713. doi: 10.1002/hep.510240339. [DOI] [PubMed] [Google Scholar]
- 19.Muller R, Baumgarten R, Markus R, Schults M, Wittenburger H, Hintsche-Kilger B, Fengler J D, Von Wussow P, Meisel H, Klein H, Malmus K, Schmidt F W. Treatment of chronic hepatitis B with interferon alfa-2b. J Hepatol. 1990;11:s137–s140. doi: 10.1016/0168-8278(90)90181-p. [DOI] [PubMed] [Google Scholar]
- 20.Munch-Petersen B, Cloos L, Tyrsted G, Eriksson S. Diverging substrate specificity of pure human thymidine kinases 1 and 2 against antiviral dideoxynucleosides. J Biol Chem. 1991;266:9032–9038. [PubMed] [Google Scholar]
- 21.Pai S B, Liu S H, Zhu Y L, Chu C K, Cheng Y C. Inhibition of hepatitis B virus by a novel l-nucleoside, 2′-fluoro-5-β-l-arabinofuranosyl uracil. Antimicrob Agents Chemother. 1996;40:380–386. doi: 10.1128/aac.40.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker W B, Cheng Y C. Mitochondrial toxicity of antiviral nucleoside analogs. J NIH Res. 1994;6:57–61. [Google Scholar]
- 23.Summers J, Mason W S. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell. 1982;29:403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- 24.Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. [PubMed] [Google Scholar]
- 25.Tipples G A, Ma M M, Fischer K P, Bain V G, Kneteman N M, Tyrrell D L J. Mutation in HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivo. Hepatology. 1996;24:714–717. doi: 10.1002/hep.510240340. [DOI] [PubMed] [Google Scholar]
- 26.Voytek P, Chang P K, Prusoff W H. Purification of deoxythymidine kinase by preparative disc gel electrophoresis and the effects of various halogenated nucleoside triphosphate on its enzymatic activity. J Biol Chem. 1971;246:1432–1438. [PubMed] [Google Scholar]
- 27.Yao G Q, Liu S H, Chou E, Kukhanova M, Chu C K, Cheng Y C. Inhibition of Epstein-Barr virus replication by a novel-l-nucleoside, 2′-fluoro-5-β-l-arabinofuranosyluracil. Biochem Pharmacol. 1996;51:941–947. doi: 10.1016/0006-2952(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 28.Yao G C, Tsai C H, Cheng Y C. Characterization of sublines of Epstein-Barr virus producing HR-1 cells and its implication in virus propagation in culture. Virus Genes. 1995;9:247–255. doi: 10.1007/BF01702880. [DOI] [PubMed] [Google Scholar]
- 29.Zoulim F, Dannaoui E, Borel C, Hantz O, Lin T-S, Liu S-H, Trepo C, Cheng Y-C. 2′,3′-Dideoxy-β-l-5-fluorocytidine inhibits duck hepatitis B virus reverse transcription and suppresses viral DNA synthesis in hepatocytes, both in vitro and in vivo. Antimicrob Agents Chemother. 1996;40:448–453. doi: 10.1128/aac.40.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoulim, F., S. Aguesse, C. Trepo, M. Chevalier, and Y. C. Cheng. Personal communication.