Abstract
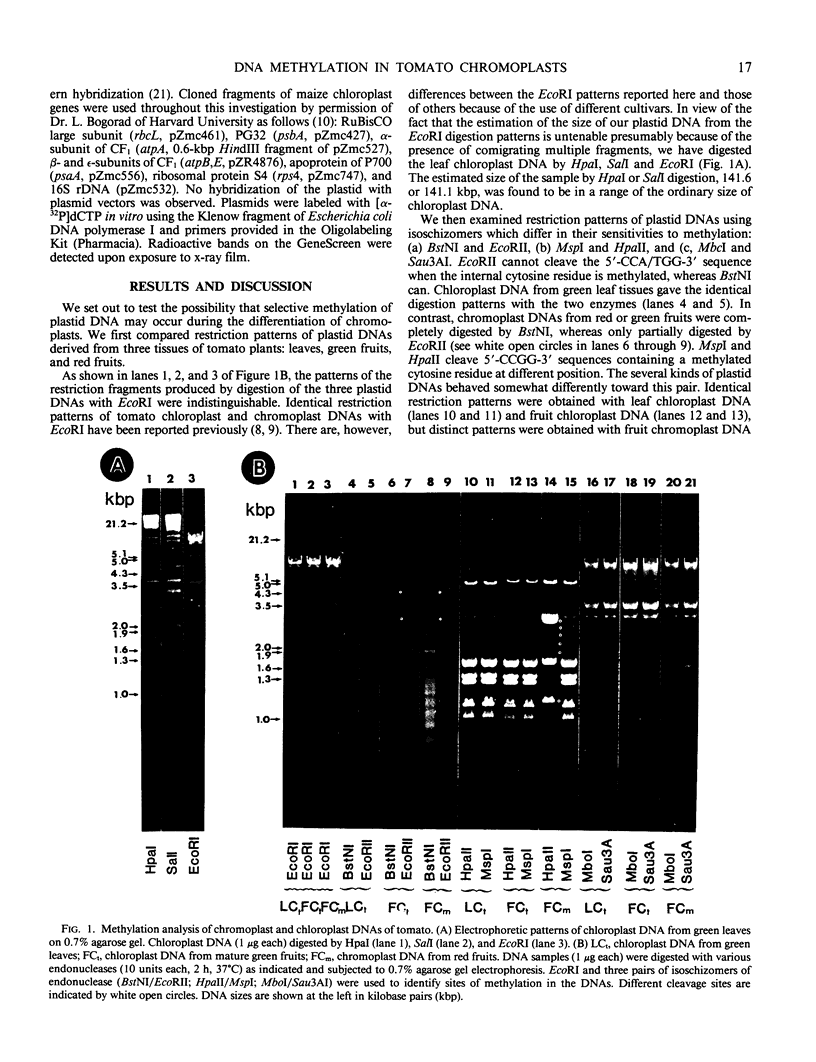
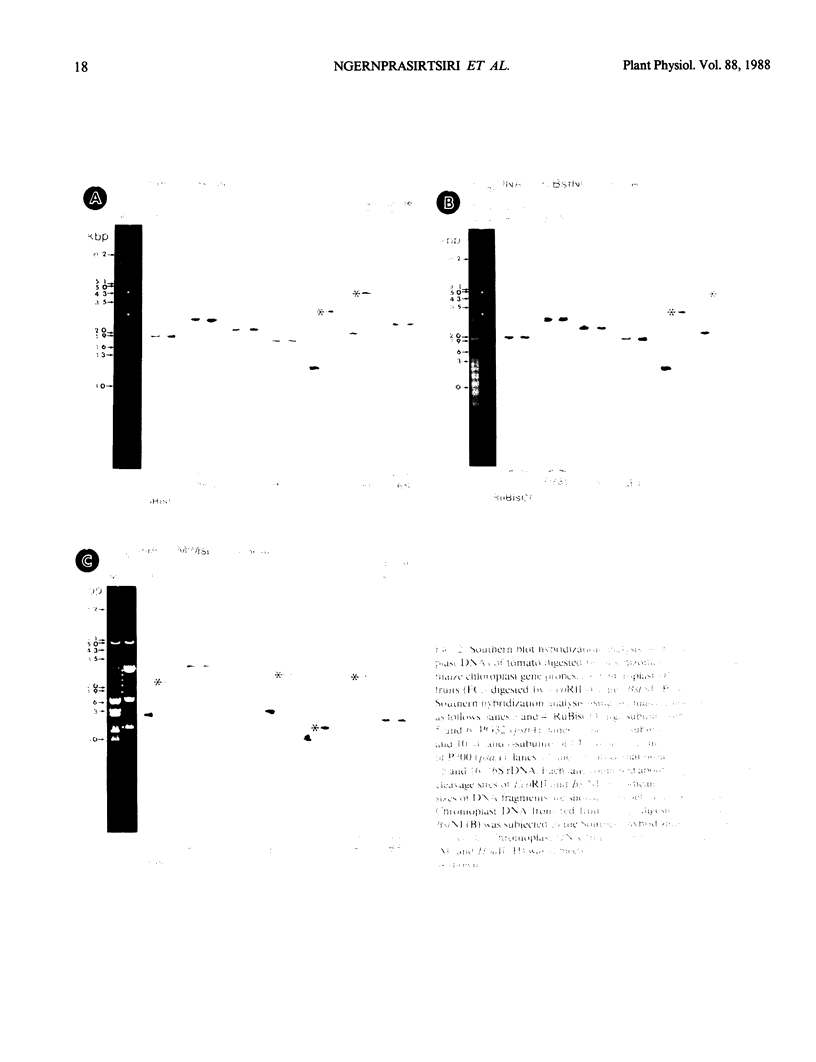
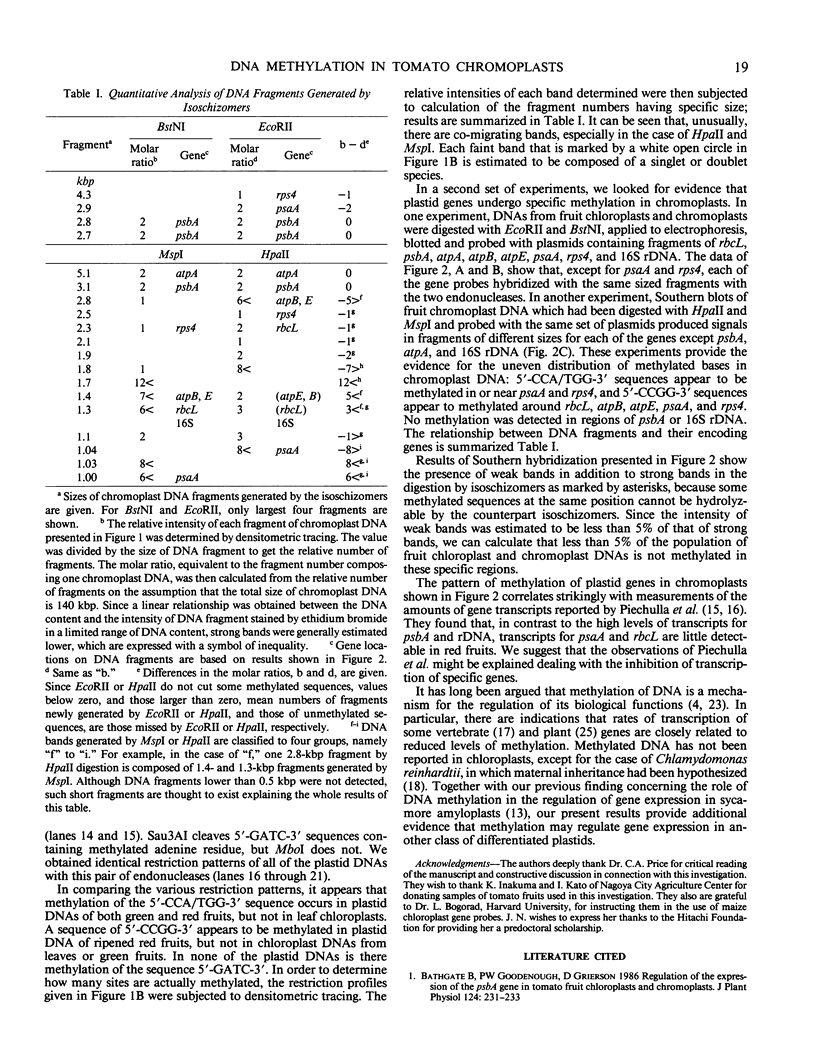
We have analyzed DNA methylation of plastid DNA from fully ripened red fruits, green mature fruits, and green leaves of tomato (Lycopersicon esculentum var. Firstmore). Essentially identical restriction profiles were obtained between chromoplast and chloroplast DNAs by EcoRI digestion. BstNI/EcoRII and HpaII/MspI are pairs of isoschizomers that can discriminate between methylated and unmethylated DNAs. These endonucleases produced different restriction patterns of plastid DNAs from tomato fruits compared to tomato leaves. Moreover, we have found from Southern blots that methylation was not detected in DNA fragments containing certain genes that are actively expressed in chromoplasts, whereas DNA fragments bearing genes that are barely transcribed in chromoplasts are methylated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Hunt C. M., Hardison R. C., Boyer C. D. Restriction enzyme analysis of tomato chloroplast and chromoplast DNA. Plant Physiol. 1986 Dec;82(4):1145–1147. doi: 10.1104/pp.82.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Bogorad L., Miles C. D. Nuclear gene-regulated expression of chloroplast genes for coupling factor one in maize. Plant Physiol. 1987 Nov;85(3):757–767. doi: 10.1104/pp.85.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherel D., Kobayashi H., Akazawa T., Kawano S., Kuroiwa T. Amyloplast nucleoids in sycamore cells and presence in amyloplast DNA of homologous sequences to chloroplast genes. Biochem Biophys Res Commun. 1985 Nov 27;133(1):140–146. doi: 10.1016/0006-291x(85)91852-2. [DOI] [PubMed] [Google Scholar]
- Ngernprasirtsiri J., Macherel D., Kobayashi H., Akazawa T. Expression of Amyloplast and Chloroplast DNA in Suspension-Cultured Cells of Sycamore (Acer pseudoplatanus L.). Plant Physiol. 1988 Jan;86(1):137–142. doi: 10.1104/pp.86.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Cedar H. DNA methylation in eukaryotic cells. Int Rev Cytol. 1984;92:159–185. doi: 10.1016/s0074-7696(08)61327-3. [DOI] [PubMed] [Google Scholar]
- Sager R., Lane D. Molecular basis of maternal inheritance. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2410–2413. doi: 10.1073/pnas.69.9.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelius A. S., Selstam E. Localization of Galactolipid Biosynthesis in Etioplasts Isolated from Dark-Grown Wheat (Triticum aestivum L.). Plant Physiol. 1984 Dec;76(4):1041–1046. doi: 10.1104/pp.76.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Watson J. C., Kaufman L. S., Thompson W. F. Developmental regulation of cytosine methylation in the nuclear ribosomal RNA genes of Pisum sativum. J Mol Biol. 1987 Jan 5;193(1):15–26. doi: 10.1016/0022-2836(87)90622-x. [DOI] [PubMed] [Google Scholar]
- Zhu Y. S., Merkle-Lehman D. L., Kung S. D. Light-induced transformation of amyloplasts into chloroplasts in potato tubers. Plant Physiol. 1984 May;75(1):142–145. doi: 10.1104/pp.75.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]