Abstract
BACKGROUND
Aneurysm formation is a complication of moyamoya disease (MMD). Distal anterior cerebral artery (ACA) aneurysms account for approximately 1% of MMD-related aneurysms. We report a case of target bypass for adult patients with MMD who presented with intracranial hemorrhage due to rupture of a distal ACA aneurysm, whose disappearance was confirmed postoperatively.
OBSERVATIONS
A 45-year-old woman presented with sudden-onset headache and loss of consciousness. Head computed tomography showed hemorrhage in the genu of the corpus callosum with intraventricular extension. Digital subtraction angiography (DSA) revealed Suzuki stage III MMD and a left A3 segment aneurysm. Superficial temporal artery (STA)–middle cerebral artery (MCA) bypass and STA-ACA target bypass were performed to reduce hemodynamic stress on the left ACA. DSA 6 months after surgery showed patency of both bypasses and disappearance of the aneurysm. At the 20-month follow-up, the patient was asymptomatic and neurologically intact.
LESSONS
Bypass revascularization may be an effective treatment to reduce hemodynamic stress and eliminate MMD-related aneurysms.
Keywords: moyamoya disease, aneurysm, revascularization, anastomosis, target bypass
ABBREVIATIONS: ACA = anterior cerebral artery, ACoA = anterior communicating artery, CT = computed tomography, DSA = digital subtraction angiography, MMD = moyamoya disease, STA = superficial temporal artery
Aneurysm formation is a complication of moyamoya disease (MMD). Cerebral hemorrhage in patients with MMD can be caused by aneurysm rupture and rupture of fragile moyamoya vessels.1–3 The estimated prevalence of cerebral aneurysms in patients with MMD ranges between 3.3% and 12.9%.4,5 Aneurysms of the distal anterior cerebral artery (ACA) account for approximately 1% of all MMD-related aneurysms.6 Patients with MMD with aneurysms generally require cerebral revascularization in addition to aneurysm treatment. However, the optimal treatment strategy for this population remains unresolved.7 Furthermore, these patients frequently have bilateral MMD, which can make treatment decisions difficult. We report an adult patient with MMD who presented with a ruptured distal ACA aneurysm and was treated with a target bypass, which resulted in the disappearance of the aneurysm.
Illustrative Case
A 45-year-old woman presented with sudden-onset headache and loss of consciousness. Head computed tomography (CT) showed hemorrhage in the genu of the corpus callosum with intraventricular extension (Fig. 1A and B). Digital subtraction angiography (DSA) showed stenosis of the left middle cerebral artery (MCA) and the A1 segment of the ACA as well as moyamoya vessels (Fig. 2A and B). The periventricular anastomoses were of the choroidal type. Other findings included reflux from the ethmoidal artery via transdural anastomoses. She was diagnosed with Suzuki stage III MMD.8 The left ACA territory was mainly supplied by blood flow from the right ACA through the anterior communicating artery (ACoA). The ACA was different from left to right. An aneurysm was visualized on the left A3 segment, which was mainly composed of progressive blood flow from the right ACA through the ACoA, indicating hemodynamic stress to the right ACA (Fig. 2C and D). Blood flow in the MCA territory was equivalent to Powers stage II on iodoamphetamine single-photon emission computed tomography. To reduce hemodynamic stress on the left ACA, we elected to perform superficial temporal artery (STA)-MCA bypass and STA-ACA bypass.
FIG. 1.

Axial (A) and coronal (B) CT scans of the head showed intracerebral hemorrhage in the genu of the corpus callosum with intraventricular perforation.
FIG. 2.
Left internal carotid artery (ICA) angiography, anteroposterior (A) and lateral (B) views, showed the left MCA, ACA A1 segment collapse (black arrows), and reflux from a transdural anastomosis from the ethmoidal artery (black arrowheads). Right ICA angiography anteroposterior view (C), lateral view (D), and 3-dimensional view (E) showed an aneurysm was formed in the left ACA A3 segment (white arrows). The left ACA area was mainly composed of progressive blood flow from the right ACA through the ACoA, which indicated hemodynamic stress to the right ACA (white arrowhead).
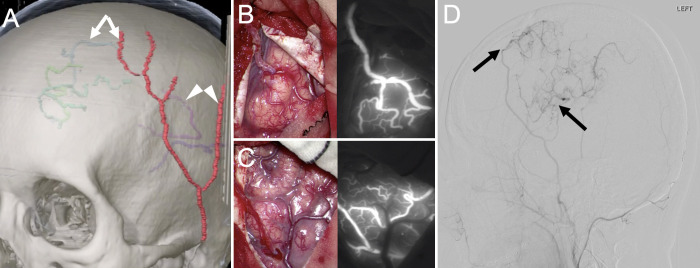
Before surgery, the STA and targeted cortical segments of the MCA and ACA on the left were identified on 3-dimensional CT angiography. Navigation mapping was used to determine the site and size of the planned craniotomies (Fig. 3A). A curved frontotemporal skin incision, which preserved the parietal and frontal branches of the STA, was made. The craniotomy for the STA-MCA bypass was performed first, and the parietal branch of the STA was anastomosed to the MCA in an end-to-side fashion (Fig. 3B). Then, after confirming the location of the targeted vessel using the navigation system, a second craniotomy was performed. Next, the frontal branch of the STA was anastomosed to the targeted vessel in the same manner (Fig. 3C). The patency of both bypasses was confirmed using indocyanine green videoangiography and Doppler ultrasonography. The dura mater was then closed with frontalis fascia and periosteal autograft, and encephalomyoarteriosynangiosis was performed. Intraoperative monitoring exhibited no changes during the operation.
FIG. 3.
A: Preoperatively, the left STA parietal branch and left MCA (white arrowheads), left STA frontal branch, and left ACA cortical segment (white arrows) were identified on 3-dimensional CT angiography, and navigation mapping was used to determine the craniotomy area based on the location of the actual vessels. B: A small craniotomy was performed for the STA-MCA bypass, and the STA parietal branch and MCA were anastomosed with end- to-side anastomosis (left), with the patency of both bypasses confirmed on indocyanine green videoangiography (right). C: The STA frontal branch was anastomosed to the ACA cortical segment in the same manner (left), with the patency of both bypasses confirmed on indocyanine green videoangiography (right). D: Left external carotid artery angiography, lateral view, on day 7 showed bilateral patency of the bypass (black arrows).
After awakening from surgery on the following day, the patient was neurologically intact. Magnetic resonance imaging showed no new cerebral infarction, and both bypasses appeared patent. DSA on day 7 confirmed the patency of both (Fig. 3D). On DSA 6 months later, the ACA aneurysm had disappeared; both bypasses remained patent (Fig. 4). At the 20-month follow-up, the patient was asymptomatic and neurologically intact.
FIG. 4.
A–C: DSA performed 6 months postoperatively. Right ICA angiography anterior-posterior view, lateral view, and 3-dimensional view showed disappearance of the aneurysm (white arrows) due to the improved left-right difference in blood flow within the ACA (white arrowhead).
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
Cerebral aneurysms associated with MMD can occur on major arterial trunks or peripheral arteries. Major trunk aneurysms are commonly located in the vertebrobasilar system and are thought to be true aneurysms. Peripheral aneurysms are frequently found on the anterior choroidal artery, posterior choroidal artery, and posterior cerebral artery and are generally considered pseudoaneurysms. Although the rupture of major trunk aneurysms causes subarachnoid hemorrhage, a ruptured peripheral aneurysm typically causes intracerebral hemorrhage near the ventricle or intraventricular hemorrhage.7,9
It is often considered necessary to treat these aneurysms with surgical interventions. Direct surgical procedures such as aneurysmectomy or neck clipping could be effective; however, the fragile moyamoya vessels with pathophysiological problems, as well as perforators, may pose obstacles to the approach. Additionally, the distal location of these aneurysms increases the difficulty of localization. Therefore, surgical clipping is not recommended in all the patients. Spontaneous aneurysm resolution and resolution after revascularization surgery have been reported.7,9,10 However, some will rupture multiple times without treatment. One possible reason for the spontaneous resolution of a ruptured aneurysm with bypass is that the bypass altered the hemodynamics and reduced the load on the aneurysm. Therefore, it is suggested that the aneurysm in this case may have been a pseudoaneurysm.
Table 1 summarizes our patient and 2 others with surgically treated MMD-related ACA aneurysms who were described in previous reports.11,12 Two presented with aneurysm rupture and intracranial hemorrhage. One had bilateral MMD. The aneurysm locations were A1, A1–A2 junction, and distal ACA, respectively. The maximum diameter ranged from 2.3 to 5.7 mm. One was treated with endovascular embolization and 2 with revascularization. Although all 3 aneurysms resolved, the reported follow-up for the embolization patient was only 14 days.
TABLE 1.
Summary of intracranial aneurysms in MMD treated with surgical approach
| Authors & Year | Age (yrs) | Sex | Diagnosis | Type of MMD | Aneurysm Location | Max Diameter (mm) | Treatment Strategy | FU | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Amin-Hanjani et al., 201413 |
43 |
F |
SAH |
Bilat |
Distal thalamostriate branch |
3 |
STA-MCA anastomosis after coil embolization |
6 mos |
No regrowth |
| Kanamori et al., 201814 |
41 |
F |
IVH |
Unilat |
Choroidal artery territory |
NA |
STA-MCA anastomosis, EMS, & EDAS |
12 mos |
No regrowth |
| 35 |
M |
IVH |
Unilat |
Lat pst choroidal artery |
NA |
STA-MCA anastomosis & EMS |
8 mos |
No regrowth |
|
| Noureldine et al., 202012 |
14 |
F |
SAH |
Bilat |
A1 segment |
5.7 |
Coiling |
14 days |
No regrowth |
| Nakajima et al., 202211 |
4 |
M |
UA |
Unilat |
A1–A2 junction |
3.9 |
ACA-ACA anastomosis |
24 mos |
No regrowth |
| Present case | 45 | F | ICH | Unilat | A3 segment | STA-A4 anastomosis | 12 mos | No regrowth |
EDAS = encephaloduroarteriosynangiosis; EMS = encephalomyosynangiosis; FU = follow-up; ICH = intracerebral hemorrhage; IVH = intraventricular hemorrhage; NA = not applicable; pst = posterior; SAH = subarachnoid hemorrhage; UA = unruptured aneurysm.
Table 1 also summarizes 3 cases with surgically treated MMD-related aneurysms in other locations that were described in previous reports.13,14 In all cases, aneurysms were identified at the onset of hemorrhage, but all patients have done well with bypass surgery and synangiosis. Another retrospective cohort study showed that of 15 aneurysms that underwent revascularization, 12 of 13 peripheral aneurysms were obliterated during the follow-up period, and 1 remained stable. Of the 2 posterior main trunk aneurysms, 1 remained stable and the other decreased in size. There have been no reports of regrowth.15
Surgical clipping of MMD-related cerebral aneurysms is difficult because moyamoya vessels generally obstruct the approach, and these vessels are easily damaged because of their fragility.11 Although endovascular embolization of a true aneurysm can achieve a good result,12 embolizing a pseudoaneurysm may result in rehemorrhage and regrowth. In our patient, we elected to perform both STA-MCA and STA-ACA target bypass to reduce the hemodynamic stress on the left ACA. This stress was thought to be the cause of the patient’s aneurysm and rupture.
The rehemorrhage rate in adults with hemorrhagic MMD is high, and the prognosis is poor.16 In the Japan Adult Moyamoya Trial, patients with posterior circulation hemorrhage were at higher risk for recurrent hemorrhage and received a greater benefit from surgical treatment than those with anterior circulation hemorrhage.17 However, hemorrhage specifically associated with MMD-related aneurysms has not been adequately investigated. The indications for direct revascularization in patients with MMD with ruptured anterior circulation aneurysms have not been established. Further studies are warranted.
Lessons
Bypass revascularization may be an effective treatment to reduce hemodynamic stress and eliminate MMD-related aneurysms.
Acknowledgments
We thank Edanz for editing a draft of the manuscript.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Oda, Kusakabe, Kobayashi, Yoshinaga, Takemoto, Abe. Acquisition of data: Oda, Kusakabe, Yoshinaga, Takemoto. Analysis and interpretation of data: Oda, Kusakabe, Yoshinaga. Drafting the article: Oda, Kusakabe, Yoshinaga, Fukumoto, Takemoto, Morishita, Abe. Critically revising the article: Fukumoto, Takemoto, Morishita, Abe. Reviewed submitted version of manuscript: Fukumoto, Morishita, Abe. Approved the final version of the manuscript on behalf of all authors: Oda. Administrative/technical/material support: Kawano. Study supervision: Kobayashi, Morishita, Abe.
References
- 1. Kim JH, Kwon TH, Kim JH, Chong K, Yoon W. Intracranial aneurysms in adult moyamoya disease. World Neurosurg. 2018;109:e175–e182. doi: 10.1016/j.wneu.2017.09.127. [DOI] [PubMed] [Google Scholar]
- 2. Yeon JY, Kim JS, Hong SC. Incidental major artery aneurysms in patients with non-hemorrhagic moyamoya disease. Acta Neurochir (Wien) 2011;153(6):1263–1270. doi: 10.1007/s00701-011-0948-y. [DOI] [PubMed] [Google Scholar]
- 3. Zhang L, Xu K, Zhang Y, Wang X, Yu J. Treatment strategies for aneurysms associated with moyamoya disease. Int J Med Sci. 2015;12(3):234–242. doi: 10.7150/ijms.10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takahashi JC, Miyamoto S. Moyamoya disease: recent progress and outlook. Neurol Med Chir (Tokyo) 2010;50(9):824–832. doi: 10.2176/nmc.50.824. [DOI] [PubMed] [Google Scholar]
- 5. Kim S, Jang CK, Park EK, et al. Clinical features and outcomes of intracranial aneurysm associated with moyamoya disease. J Clin Neurol. 2020;16(4):624–632. doi: 10.3988/jcn.2020.16.4.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ge P, Ye X, Zhang Q, et al. Clinical features, surgical treatment, and outcome of intracranial aneurysms associated with moyamoya disease. J Clin Neurosci. 2020;80:274–279. doi: 10.1016/j.jocn.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 7. Kwak R, Ito S, Yamamoto N, Kadoya S. Significance of intracranial aneurysms associated with moyamoya disease. (Part I). Differences between intracranial aneurysms associated with moyamoya disease and usual saccular aneurysms – review of the literature. Neurol Med Chir (Tokyo) 1984;24(2):97–103. doi: 10.2176/nmc.24.97. Article in Japanese. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20(3):288–299. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- 9. Yamada H, Saga I, Kojima A, Horiguchi T. Short-term spontaneous resolution of ruptured peripheral aneurysm in moyamoya disease. World Neurosurg. 2019;126:247–251. doi: 10.1016/j.wneu.2019.02.193. [DOI] [PubMed] [Google Scholar]
- 10. Kawaguchi S, Sakaki T, Morimoto T, Kakizaki T, Kamada K. Characteristics of intracranial aneurysms associated with moyamoya disease. A review of 111 cases. Acta Neurochir (Wien) 1996;138(11):1287–1294. doi: 10.1007/BF01411057. [DOI] [PubMed] [Google Scholar]
- 11. Nakajima K, Funaki T, Okawa M, Yoshida K, Miyamoto S. Successful shrinkage of anterior communicating artery aneurysm after ACA-ACA bypass with interposed occipital artery graft in pediatric moyamoya disease: illustrative case. J Neurosurg Case Lessons. 2021;2(17):CASE21460. doi: 10.3171/CASE21460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Noureldine MHA, Saikali I, Nassif A, et al. Pediatric moyamoya presenting as a subarachnoid hemorrhage from a ruptured anterior cerebral artery aneurysm. World Neurosurg. 2020;134:123–127. doi: 10.1016/j.wneu.2019.10.157. [DOI] [PubMed] [Google Scholar]
- 13. Amin-Hanjani S, Goodin S, Charbel FT, Alaraj A. Resolution of bilateral moyamoya associated collateral vessel aneurysms: rationale for endovascular versus surgical intervention. Surg Neurol Int. 2014;5(Suppl 4):S155–S160. doi: 10.4103/2152-7806.134812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanamori F, Takasu S, Ota S, Seki Y. Prevention of the rerupture of collateral artery aneurysms on the ventricular wall by early surgical revascularization in moyamoya disease: report of two cases and review of the literature. World Neurosurg. 2018;109:393–397. doi: 10.1016/j.wneu.2017.10.059. [DOI] [PubMed] [Google Scholar]
- 15. Ni W, Jiang H, Xu B, et al. Treatment of aneurysms in patients with moyamoya disease: a 10-year single-center experience. J Neurosurg. 2018;128(6):1813–1822. doi: 10.3171/2017.3.JNS162290. [DOI] [PubMed] [Google Scholar]
- 16. Miyamoto S, Yoshimoto T, Hashimoto N, et al. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the Japan Adult Moyamoya Trial. Stroke. 2014;45(5):1415–1421. doi: 10.1161/STROKEAHA.113.004386. [DOI] [PubMed] [Google Scholar]
- 17. Takahashi JC, Funaki T, Houkin K, et al. Significance of the hemorrhagic site for recurrent bleeding: prespecified analysis in the Japan adult moyamoya trial. Stroke. 2016;47(1):37–43. doi: 10.1161/STROKEAHA.115.010819. [DOI] [PubMed] [Google Scholar]