Abstract
In terrestrial ecosystems, leaf litter is the main source of nutrients returning to the soil. Understanding how litter decomposition responds to stand age is critical for improving predictions of the effects of forest age structure on nutrient availability and cycling in ecosystems. However, the changes in this critical process with stand age remain poorly understood due to the complexity and diversity of litter decomposition patterns and drivers among different stand ages. In this study, we examined the effects of stand age on litter decomposition with two well-replicated age sequences of naturally occurring secondary forests and Chinese fir (Cunninghamia lanceolata) plantations in southern China. Our results showed that the litter decomposition rates in the secondary forests were significantly higher than those in the Chinese fir plantations of the same age, except for 40-year-old forests. The litter decomposition rate of the Chinese fir initially increased and then decreased with stand age, while that of secondary forests gradually decreased. The results of a structural equation model indicated that stand age, litter quality and microbial community were the primary factors driving nutrient litter loss. Overall, these findings are helpful for understanding the effects of stand age on the litter decomposition process and nutrient cycling in plantation and secondary forest ecosystems.
Subject terms: Forest ecology, Forestry
Introduction
In terrestrial ecosystems, leaf litter is the main source of nutrients returning to the soil1–3. Litter decomposition plays a crucial role in regulating nutrient cycling and organic matter turnover within forest ecosystems4,5. At a local scale, litter quality and decomposers are the key factors influencing litter decomposition and nutrient release6–8. As forest stand age progresses, the microclimate, soil properties, vegetation composition and microbial community undergo changes, exerting a significant influence on litter decomposition and nutrient release9–11. Previous studies have shown that the decomposition rate of litter will not change monotonically due to the ratio of litter carbon to nitrogen first increasing and then decreasing with stand age12,13. However, other studies have indicated that the litter decomposition rate decreased gradually as the initial carbon content of litter increased with stand age14. These inconsistent results highlight the complexity of changing patterns and driving factors of litter decomposition across different stand ages14. Therefore, exploring the dynamics and mechanisms of litter decomposition at various stand ages is crucial for gaining a comprehensive understanding of nutrient cycling in forest ecosystems.
Litter microorganisms and their activities are critical to litter decomposition9,15,16. The microbial community and enzyme activity within litter are highly sensitive to changes in nutrient availability and vegetation diversity that occur as forests age10,17. Evidence shows that both the microbial community and enzyme activity within litter tend to increase with stand age17,18. Moreover, studies have demonstrated that the microbial communities and enzyme activity within litter exhibit responses to variations in plant diversity and composition that arise from forest age10,19. In addition, the abundance and diversity of microorganisms tend to increase as forests age due to the relative humidity increasing during forest succession20.
In southern China, naturally occurring secondary forests and Chinese fir plantations are the dominant forest types, with cover areas reaching over 138 million ha and 11 million ha, respectively21,22. For our study, we established long-term age sequences in both Chinese fir plantations and naturally occurring secondary forests, with three replicates for each forest age. A two-year field litter decomposition experiment was conducted to evaluate the effects of litter quality and microbial changes with stand age on litter decomposition and nutrient release in the different forest types. We hypothesized that (1) compared to Chinese fir plantations, naturally occurring secondary forests would exhibit a higher litter decomposition rate and nutrient release than of the same age stage, and this scenario would be attributed to the higher litter nitrogen content in natural secondary forests. (2) The decomposition rate of litter would reach its peak at the middle stand age. Relative humidity and nutrient availability increase with stand age and provide favourable conditions for microbial richness and diversity, ultimately promoting litter decomposition10,20,23. (3) The nutrient release rate of a middle-aged forest is faster than that of a young forest and a mature forest, especially in the early stage of litter decomposition. Middle-aged forests with higher litterfall production and lower carbon–nitrogen ratio will accelerate the release of litter nutrients24–26.
Results
Dynamics of litter mass, C and N loss
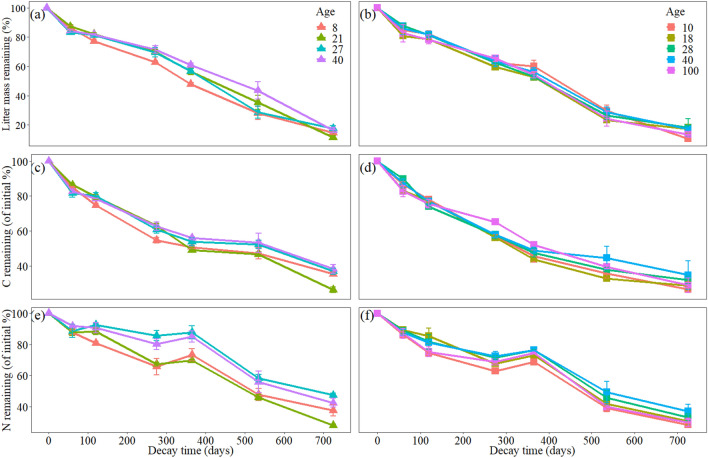
At the early stage of the decomposition experiment, both Chinese fir and naturally occurring secondary forest exhibited rapid litter mass loss, with litter remaining at approximately 83.27–87.35% for Chinese fir and 80.5–87.43% for naturally occurring secondary forest at 60 days (Fig. 1a,b). Over the subsequent two months, the litter mass loss in both Chinese fir and naturally occurring secondary forest slowed. In the other decomposition stages, the rate of litter mass remaining continued to decline with fluctuations (Fig. 1a,b). At 275 and 365 days, the litter mass remaining in CF8 was significantly lower than that in the other Chinese fir stands (P < 0.05). At 535 days, both CF8 and CF27 had significantly lower litter mass remaining compared to that in CF40 (P < 0.05). At 275 days, the litter mass remaining in the primary forests (NF100) was significantly higher than that in NF10 (P < 0.05). At 365 days, the litter mass remaining in NF18 and NF28 was significantly lower than that in NF10 (P < 0.05).
Figure 1.
Litter mass, C and N remaining in litter from different stand age classes of Chinese fir (a,c,e) and naturally occurring secondary forest (b,d,f). Values represent the mean ± se.
The temporal pattern of litter carbon (C) and nitrogen (N) loss depended on forest type and stand age (Table 1). Specifically, in the Chinese fir stands, compared to CF27 and CF40, CF21 exhibited significantly faster leaf litter C and N loss (Fig. 1c,e). The leaf litter C and N losses of NF10 were significantly higher than those of NF18, NF28 and NF38 (Fig. 1d,f). In the middle stage of decomposition, there was an increase in the proportion of remaining N to initial nitrogen, suggesting slight net N immobilization in both Chinese fir and naturally occurring secondary forest (Fig. 1e,f).
Table 1.
Results of a three-way repeated-measures ANOVA (F and P values) testing for the effects of forest type, stand age, decomposition time and their interactions on mass loss and leaf litter C and N loss from litterbags decomposed in soils in the Chinese fir and naturally occurring secondary forest stands.
| Variation | df | Mass loss (%)a | kb | Litter C lossa | Litter N lossb | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | ||
| Forest type (F) | 1 | 130.87 | < 0.001 | 75.523 | < 0.001 | 104.318 | < 0.001 | 166.884 | < 0.001 |
| Stand age (A) | 7 | 5.58 | < 0.001 | 4.528 | < 0.001 | 3.684 | 0.001 | 19.292 | < 0.001 |
| Time (T) | 6 | 1865.39 | < 0.001 | 255.057 | < 0.001 | 1059.478 | < 0.001 | 889.353 | < 0.001 |
| F × A | 8 | 0.333 | 0.952 | 1.361 | 0.217 | 0.441 | 0.895 | 1.204 | 0.299 |
| F × T | 6 | 16.58 | < 0.001 | 4.767 | < 0.001 | 14.963 | < 0.001 | 10.215 | < 0.001 |
| A × T | 42 | 1.72 | 0.011 | 2.085 | 0.001 | 1.645 | 0.018 | 1.96 | 0.003 |
| F × A × T | 62 | 186 | < 0.001 | 28.17 | < 0.001 | 107.2 | < 0.001 | 101.48 | < 0.001 |
aPlot was not a significant random factor and was dropped from the model.
bPlot was a significant random factor and included in the model.
Litter decomposition rate
The litter decomposition rates of the Chinese fir forest first increased and then decreased with stand age, whereas those of the naturally occurring secondary forest first decreased and then increased with stand age (Table 2). The decomposition rates of the naturally occurring secondary forest were significantly higher than those of Chinese fir forest, excluding the 40-year-old forest (P < 0.05). Among the Chinese fir stands, the litter decomposition rates in CF21 were significantly higher than those in CF8, CF27 and CF40 (P < 0.05). In the naturally occurring secondary forest, the decomposition rates in NF10, NF18 and NF100 were significantly higher than those in NF38 (P < 0.05).
Table 2.
Models (y = ae−kt) for the relationship between mass remaining (y, %) of Chinese fir and naturally occurring secondary forest leaf litter and time (t, a).
| Regression equation | Decomposition constant (g g−1 a−1) | R2 | T50(a) | |
|---|---|---|---|---|
| CF8 | y = 97.72e−0.581t | 0.581Bb | 0.991 | 1.15 |
| CF21 | y = 105.15e−0.683t | 0.683Ba | 0.969 | 1.09 |
| CF27 | y = 98.1e−0.539t | 0.539Bc | 0.981 | 1.26 |
| CF40 | y = 99.26e−0.538t | 0.538Abc | 0.977 | 1.27 |
| NF10 | y = 105.46e−0.872t | 0.872Aa | 0.982 | 0.87 |
| NF18 | y = 101.45e−0.832t | 0.832Aab | 0.973 | 0.85 |
| NF28 | y = 103.51e−0.762t | 0.762Abc | 0.992 | 0.96 |
| NF38 | y = 98.58e−0.609t | 0.609Ac | 0.995 | 1.12 |
| NF100 | y = 104.56e−0.823t | 0.823ab | 0.965 | 0.9 |
T50(a) represents the time required for a 50% mass loss for the litter from different forest age sequences. The uppercase letters indicate significant differences under two forest types of similar forest age, while the lowercase letters indicate significant differences under different stand ages of the same forest type (P < 0.05).
Changes in litter microbial community composition
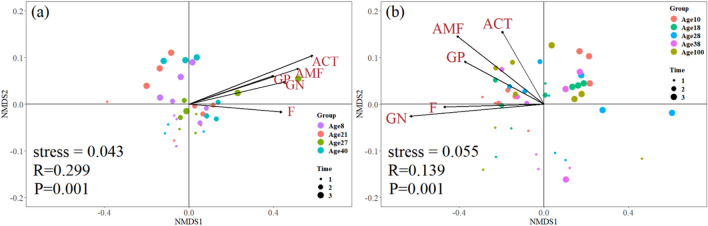
The composition of the microbial community within the litter exhibited significant differences throughout the decomposition process, showing dispersion effects of different forest types and stand ages at the beginning of decomposition, and they gradually converged at the later stage of litter decomposition (Fig. 2). Both Chinese fir and naturally occurring secondary forest displayed a dominance of fungi at the early stages, while the proportions of gram-positive bacteria, gram-negative bacteria, arbuscular mycorrhizal fungi, fungi and actinomycetes increased at the middle stage (Fig. 2). However, the composition of the microbial community converged with the decomposition process for leaf litter from the Chinese fir and naturally occurring secondary forest stands (Fig. 2).
Figure 2.
Nonmetric multidimensional scaling (NMDS) ordination of microbial community composition for Chinese fir (a) and naturally occurring secondary forest (b) during the decomposition period. The groups represent the stand age of Chinese fir and natural secondary forest; the time represents sampling dates (on the 0, 365th and 725th days). Stress Stress function in groups, R Similarities of microbial community, P Significance of similarity analysis of microbial community at different times, GP Gram-positive bacteria, GN Gram-negative bacteria, F Fungi, AMF Arbuscular mycorrhizal fungi, ACT Actinomycetes.
Factors that control the litter decomposition rate, mass and nutrient loss
The results of the linear mixed-effect model indicated that litter decomposition was synergistically controlled by forest age, soil environment, litter quality, and microorganisms. The results of the optimal linear mixed effect model for the litter decomposition rate indicated that the litter decomposition rate was mainly regulated by the contents of soil N, litter N, lignin, cellulose and β-N-acetyl-glucosaminidase (NAG) and the F: (GP + GN) ratio (P < 0.05, Supplementary Table 1). The combination of the above factors with cellobiohydrolase (CBH), arbuscular mycorrhizal fungi (AMF) and soil pH explained 37% of the variation in the litter decomposition rate. Moreover, the results of the optimal linear mixed effect model demonstrated that litter mass and nutrient loss are mainly regulated by the soil environment, litter quality, microbial community and their interaction. The ratio of soil C:N and total PLFAs showed a significantly positive effect on the remaining leaf litter mass, while litter N, lignin:litter N, AMF, fungi and GP had a significantly negative effect (P < 0.05, Supplementary Table 2). The litter C, NAG and F:(GP + GN) ratio showed a significantly negative effect on litter C loss, whereas the litter N, cellulose, lignin, AMF, fungi and SM showed a significantly positive effect (P < 0.05, Supplementary Table 3). The litter N, lignin, cellulose, AMF, fungi and GP:GN ratio showed a significantly positive effect on litter N loss, while the litter C, NAG, ammoniacal nitrogen () and F:(GP + GN) ratio had a significantly negative effect (P < 0.05, Supplementary Table 4).
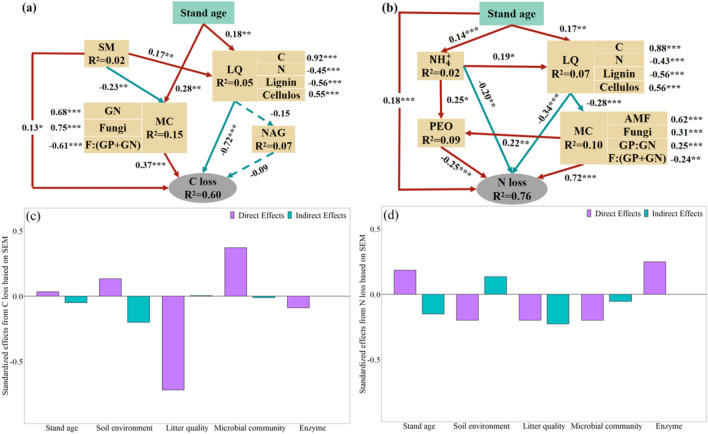
The results of structural equation modelling indicated that the soil environment, litter quality, microbial community, enzyme activity and their interactions could directly or indirectly drive litter nutrient loss (Fig. 3a,b). The litter quality and microbial community were the main factors that directly controlled litter C and N loss (P < 0.001, Fig. 3a,b). Litter quality showed a significantly negative direct effect on litter C and N loss, whereas the microbial community had a significant direct positive effect (Fig. 3a,b). At the same time, stand age and soil environment indirectly affected litter C and N loss by affecting litter quality (Fig. 3). The effect of litter enzyme activity on litter C and N loss was not significant.
Figure 3.
Structural equation model showing the effects of stand age, soil environment, soil nutrients, litter quality, microbial community and enzyme activity on litter C and N loss (N = 189). (a,b) Path diagrams of factors influencing litter C and N stocks. (a,b) Summed direct and indirect effects. Solid red and green arrows represent positive and negative relationships, respectively. Dashed arrows represent no significant effects. Numbers adjacent to arrows are standardized path coefficients, analogous to relative regression weights. (***P < 0.001; **P < 0.01; *P < 0.05). R2 represents the proportion of variance explained for every response variable in the model. The goodness-of-fit statistics for panels (a,b) are P = 0.306, GFI = 0.98, RMSEA = 0.027 and P = 0.421, GFI = 0.974, RMSEA = 0.013, respectively. SE Soil environment, LQ Litter quality, MC Microbial community, EN Enzyme, SM Soil moisture, C Litter C, N Litter N, GN Gram-negative bacteria, F:(GP + GN) Fungal-bacterial ratio, AMF Arbuscular mycorrhizal fungi, NAG β-N-acetyl-glucosaminidase, PEO Peroxidase.
Discussion
Our results showed that the litter decomposition rate of the secondary forest was significantly higher than that of the Chinese fir plantation at the same age, except for the 40-year-old forest, which was consistent with our first hypothesis. Previous studies have indicated that the decomposition rate of broad-leaved species is significantly higher than that of conifer species27–29. Our results support this conclusion due to the lower litter N content and the presence of more recalcitrant substances (i.e., lignin and cellulose) in the Chinese fir plantations (Table S5). Furthermore, as stand age increases, changes in plant species and composition have a positive effect on decomposition rates30. Previous studies at our site have suggested that understorey vegetation diversity is higher in naturally occurring secondary forests and primary forests than in Chinese fir plantations31. In addition, during the process of litter decomposition, the mixture of litter with different qualities will interact and produce positive nonadditive mixture effects, thus promoting litter decomposition32–34. These factors collectively contribute to the accelerated litter decomposition rate in naturally occurring secondary forests and primary forests.
The quality of litter exerts an important influence on the litter decomposition rate35,36. Consistent with our second hypothesis, the decomposition rate of the litter in the middle-aged Chinese fir forests was higher than that in the younger and older forests. The characteristics of litter with a higher N content and lower C:N ratio in the middle-aged forests were the main reasons for the high decomposition rate35,37. Additionally, during the process of forest development, the forest self-thinning effect enlarges the forest gap and enhances the photodegradation of litter, thereby accelerating the decomposition of litter5,38,39. Moreover, the relative humidity tends to increase in middle-aged forests with a higher diversity of understorey vegetation, which further accelerates the decomposition of litter20,31.
For the age sequence of the secondary forest, the decomposition rate of litter decreased with stand age and increased in the primary forest, which was contrary to our second hypothesis. The increasing canopy cover in a secondary forest as the forest develops and the subsequent decrease in understorey biomass can slow litter decomposition33,40,41. Previous studies conducted on the age sequences of secondary forests in tropical regions have also indicated an increase in relative humidity with stand age, accompanied by a decrease in light availability and soil temperature20. The variation in environmental factors such as forest canopy cover, vegetation type, soil temperature and soil moisture with forest stand age can reduce nonadditive mixture effects, which can hinder litter decomposition20,42. Variations in stand vegetation type, age scale and study location may account for the differences described above11,13,42. However, previous studies have shown that forest type exerts an important influence on litter decomposition42. Our findings support this notion by revealing distinct patterns of litter decomposition rates with the age sequences of secondary forests and Chinese fir forests, further highlighting the importance of forest type in litter decomposition.
In both the Chinese fir and secondary forest age sequences, the nutrient release rate of middle-aged forest was faster than young and mature forests, which was consistent with our third hypothesis. In our study, middle-aged forests with higher litterfall production and lower carbon–nitrogen ratio accelerate the release of litter nutrients24,35. Litter C and N are necessary nutrients for soil microbial activities are easily absorbed and utilized by microorganisms, and quickly released in the early stages of decomposition25. It has been well documented by several researchers that the general trend of litter decomposition is initially rapid and then slow33,43,44. The present study found that the rate of nitrogen release was faster in the younger forest, possibly due to the high demand for nitrogen by vegetation in the early stages of forest regeneration to support their growth26. Previous studies have also suggested that temporal patterns of litter C and N loss are influenced by stand age11,30, and the results of our research also support this conclusion. The results of the structural equation model showed that stand age had an indirect effect on litter C and N loss by increasing litter nitrogen and/or microbial biomass. Furthermore, lignin had a significant negative effect on the release of carbon and nitrogen from litter, which was consistent with previous studies36.
Changes in forest stand age may be paralleled by shifts in microbial community composition10,45. Our results demonstrated that there were differences in the microbial community composition at the initial stage of litter decomposition among the different stand ages of the naturally occurring secondary forests and Chinese fir, although these differences were not significant (Fig. 3). The ability to metabolize easily accessed carbon is believed to be universal to various microorganisms46. In the early stage of decomposition, easily accessed or unbound carbon accounts for a high proportion of the initial composition and cannot be a limiting factor for microbial activities47,48. At the later stage of decomposition, microbial community composition in litter converged in the two forest types with different stand ages. This finding may be attributed to the depletion of labile substrates during further decomposition or their binding with other compounds, making them less available for microbial utilization48.
Enzymatic activities are generated in response to limited substrate and nutrient availability, facilitating nutrient acquisition during decomposition9. Our results showed that the soil environment did not have a direct effect on enzyme activities. Previous studies have indicated that enzyme activity can occur over a broad temperature range or based on the production of multiple isoenzymes, each with different temperature optima but with similar affinity to the substrate49. Other studies have shown that the decoupling between enzyme activities and the soil environment may indicate that microbial growth is not primarily limited by nutrient availability50. In addition, the accumulation of microbial biomass always delays the increase in enzyme activity9, which further supports our research results.
Conclusions
In conclusion, the effects of forest stand age on litter decomposition and nutrient release dynamics in naturally occurring secondary forests and Chinese fir plantations were tested in this study. Our results suggest that forest age and forest type have a significant influence on litter quality, thus affecting nutrient cycling. The nutrient cycle of Chinese fir plantations first increased and then decreased with stand age and slowed with secondary forest succession. Furthermore, our results also showed that the nutrient turnover in the secondary forest was significantly faster than that in the Chinese fir plantations of the same age, except for the 40-year-old forest. These findings are helpful for understanding the effects of stand age on the process of litter decomposition and nutrient cycling in plantation and secondary forest ecosystems. Unfortunately, our research lacks the inclusion of older forests, which may have an impact on the research results. Therefore, it is necessary to carry out further research in forest age series containing more forest age stages.
Methods
Study area and experimental design
This experiment was conducted in naturally occurring secondary forests and Chinese fir (C. lanceolata) plantations, which are located in Baisha Forest Farm (116° 20′ 00″–116° 40′ 57″ E; 24° 52′ 01″–25° 16′ 59″ N) in Fujian Province, Southeast China. According to the Köppen climate classification, the climate is monsoon-influenced humid subtropical, with long hot and humid summers and short mild winters. The mean annual precipitation is 1646 mm, and the mean annual temperature is 20.1 °C (lowest in January, i.e., 6.7 °C; highest in July, i.e., 29.1 °C), with a frost-free period of 277 days31. The elevation ranges from 453 to 875 m. According to the IUSS Working Group WRB 2014, the soil type in the study area is Oxisol51, and the soils were developed from granite. Natural secondary forest species are mainly dominated by Castanopsis carlesii, Castanopsis fargesii, Schima Superba, Castanopsis faberi, Liquidambar formosana, Sloanea sinensis, etc.
In May 2019, we selected four stages of Chinese fir plantations and natural secondary forests, with 8-, 21-, 27-, and 40-year-old Chinese fir stands (hereafter called CF8, CF21, CF27, and CF40, respectively) and approximately 10-, 18-, 28-, and 38-year-old natural secondary forests (hereafter called NF10, NF18, NF28, and NF38, respectively). In addition, we selected primary forests (> 100 yr) that were undisturbed by humans for more than centuries (hereafter called NF100). The natural secondary forests were transformed from clear-cutting Chinese fir and Pinus massoniana plantations. There is no human intervention in the process of growth. We mainly determine the age of the secondary forest according to the logging records of the forest farm. Three replicated plots (20 × 30 m = 600 m2) were chosen for each stand age class, and replicated plots of the same stand age were at least 500 m apart. In addition, to prevent the edge effect, all experimental plots were at least 100 m away from the edge of farmland, road or other types of woodland. After the establishment of the sample plot, we investigated the stand characteristics and vegetation community composition of Chinese fir plantations and natural secondary forests with different stand ages (Table 3).
Table 3.
The stand characteristics and vegetation community composition of Chinese fir plantations and natural secondary forests with different stand ages.
| Plot | N | Stand density (stems/ha) | Mean DBH (cm) | Basal area (m2/ha) | Stand composition (% of stand basal area) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chinese fir | Castanopsis carlesii | Castanopsis fargesii | Schima superba | Castanopsis faberi | Castanopsis fissa | Othera | |||||
| CF8 | 3 | 3088 ± 804 | 12.28 ± 0.68 | 32 ± 2.95 | 97 ± 2 | 0 | 0 | 0 | 0 | 0 | 3 ± 2 |
| CF21 | 3 | 1400 ± 352 | 19.81 ± 1.01 | 40.2 ± 7 | 93 ± 3.5 | 0 | 0 | 0 | 0 | 0 | 6 ± 3.5 |
| CF27 | 3 | 1863 ± 444 | 23.22 ± 1.37 | 43.8 ± 8.45 | 96 ± 2 | 0 | 0 | 0 | 0 | 0 | 3 ± 2 |
| CF40 | 3 | 1142 ± 291 | 27.85 ± 1.94 | 43.4 ± 5.3 | 92 ± 3 | 0 | 5 ± 1 | 0 | 0 | 0 | 3 ± 1.5 |
| NF10 | 3 | 2739 ± 110 | 9.32 ± 2 | 23.2 ± 4.1 | 0 | 1 ± 0.5 | 14 ± 6 | 3 ± 1 | 0 | 20 ± 9.5 | 62 ± 14.5 |
| NF18 | 3 | 2244 ± 283 | 10.9 ± 1.4 | 25.7 ± 2.4 | 3 ± 1 | 9 ± 8 | 46 ± 3.5 | 6 ± 4 | 4 ± 3.5 | 0 | 32 ± 4.5 |
| NF28 | 3 | 2658 ± 240 | 11.1 ± 0.2 | 31.1 ± 3.7 | 2 ± 1 | 0 | 41 ± 5.5 | 10 ± 3.5 | 4 ± 4 | 4 ± 3.5 | 39 ± 5.5 |
| NF38 | 3 | 1739 ± 255 | 12.1 ± 0.6 | 27.7 ± 2.1 | 3 ± 1 | 11 ± 9.5 | 34 ± 12.5 | 26 ± 20.5 | 4 ± 4 | 0 | 22 ± 6.5 |
| NF100 | 3 | 1179 ± 109 | 16.4 ± 1.4 | 56.8 ± 6.8 | 0 | 58 ± 21 | 0 | 1 ± 0.5 | 8 ± 4.5 | 0 | 33 ± 17.5 |
The others’ category included Cinnamomum micranthum, Pinus massoniana, Sloanea sinensis, Cyclobalanopsis glauca, Cinnamomum camphora, Lithocarpus glaber, Meliosma rigida, Machilus chekiangensis, Liquidambar formosana, Cinnamomum porrectum, Micromelum falcatum, Illicium lanceolatum, Rapanea neriifolia, Engelhardtia fenzlii, Machilus versicolora, Eurya loquaiana, Rhododendron henryi, Phyllostachys heterocycle, Phoebe bournei, Ormosia xylocarpa, Castanopsis tibetana, Castanopsis eyrei, Neolitsea aurata, Adinandra millettii, Illicium ternstroemioides and Machilus velutina.
In July 2019, we collected fresh fallen litters by the litter traps and recording the plot number. Six litter traps were setted in each 20 × 30 m plot. It should be noted that the litter samples collected in this study do not include endangered species, so there are no ethical issues and other conflicts of interest. Litter samples collected from each plot were combined to represent one sample. Then, all leaf litters of each subplot were air-dried and put into litterbags (20 cm × 25 cm) made from polyvinyl screen with a mesh width = 1.5 mm. To alleviate the effect of the mesh size of the litter bag on the soil biota and insects, 16 small holes of 0.25 cm2 were evenly distributed on both sides of the litter bag to enable access for some soil biota and insects. Each litterbag was filled with 10 g of leaf litter from the Chinese fir plantations or naturally occurring secondary forests and placed on the surface of the plot corresponding to the recorded data. In each plot, 18 litterbags containing the leaf litter were tied to nylon lines to prevent losses.
Sampling and analysis
The decomposition of leaf litter started in August 2019, and two bags were randomly selected from each subplot at 0, 60, 120, 275, 365, 535, and 725 days after the start of the litter decomposition experiment. At the same time, the soil temperature (ST) and moisture (SM) were measured by a time domain reflectometer (Model TDR350, Spectrum Company, USA). In total, 378 litterbags (9 stand ages × 3 replicates × 2 litterbags × 7 sampling events) were collected and brought back to the laboratory to prepare for subsequent experiments. We carefully removed soil particles and other nontarget plant material adhering to the remaining litter. Then, we gently rinsed the remaining litter using forceps and oven-dried it at 65 °C for at least 72 h to constant weight. Subsequently, we weighed the remaining leaf litter and calculated the mass loss of litter in each sample plot. The contents of litter C and N were determined by an elemental analyser (Elementar Vario EL III, Elementar, Germany). Extraction of lignin and cellulose from the litter was conducted by the acid detergent method.
In July 2019, 0–10 cm soil samples were collected and analysed for physical and chemical properties. In each plot, 10 soil cores were randomly sampled by using a soil auger with a 5 cm diameter. All soil samples were stored in coolers and brought back to the laboratory immediately. After removing rocks and visible plant debris, the soil samples were sieved through a 2 mm mesh and mixed. All samples were divided into two subsamples, with one stored at 4 °C for dissolved organic N (DON) and mineral N determination and the other subsample air dried for analyses of soil pH, total carbon (TC) and total nitrogen (TN). Soil mineral N and DON were measured by a method in previous research52. Soil pH was determined by a glass electrode pH meter (STARTER 300, OHAUS, US) with a water to soil ratio of 2.5:152. Soil TC and TN were assessed on finely ground soil (< 0.20 mm) using an element analyser with a combustion furnace (Elementar Vario EL III, Elementar, Germany).
Litter microbial community and enzyme activity
The litter microbial community was measured by phospholipid fatty acid (PLFA) analysis and extracted only from litterbags collected at 0, 365 and 725 days after the start of the experiment because of resource constraints. We extracted phospholipids from freeze-dried litter samples according to the methods in previous studies48,53. Fatty acids were extracted from 1 g of litter samples in a one-phase solvent consisting of a 2:1:0.8 mixture of methanol, chloroform and phosphate. The phospholipid fraction was collected and transformed to fatty acid methyl esters (FAMEs) by methylation. The FAMEs were identified by gas chromatography with the MIDI Sherlock Microbial Identification System (MIDI, Inc.) and by coelution with standards. The concentrations of the PLFAs were calculated according to the internal standard concentration of 19:0. Individual PLFAs were identified using standard nomenclature. Gram-positive bacteria (GP) were assessed by i14:0, i15:0, a15:0, i16:0, i17:0 and a17:0, whereas gram-negative bacteria (GN) were represented by 16:1 w9c, 16:1 w7c, cy17:0, 18:1 w7c and 18:1 w5c54,55. Actinomycetes (ACT) were evaluated by 10Me16:0, 10Me17:0 and 10Me18:056. The fungi (F) were represented by 18:1ω9c and 18:2ω6c57,58. The arbuscular mycorrhizal fungi (AMF) were assessed by 16:1ω5c57.
Fresh litter samples for each stand age and harvesting time were used to analyse the activities of four hydrolytic enzymes, cellobiohydrolase (CBH), beta-glucosidase (BG), β-N-acetyl-glucosaminidase (NAG), and acid phosphatase (AP), and two oxidase enzymes, phenol oxidase (PHO) and peroxidase (PEO), involved in the microbial acquisition of C, N, P and refractory substances15,59. Freshly cut litter samples (1.5 g) were added to a 125 ml conical flask with 50 mmol/L acetate buffer (pH = 5) and stirred with a magnetic stirrer. The mixture was stirred for 10 min, allowed to stand, and then transferred to a clean beaker for further use. Aliquots of 200 µl of suspension were dispensed into 96-well microplates, and then, 50 µl of substrate solution was added at a concentration of 200 µmol substrate g−1 sample. Finally, the microplates were incubated at 25 °C with hydrolytic enzyme for 4 h and oxidase for 20 h. After incubation, the reaction of the hydrolytic enzyme was terminated by adding a 10 µl aliquot of NaOH to each well9. The enzyme activities were measured by a multifunction microplate reader (Spectra Max M5, Molecular Devices, USA).
Litter decomposition rate
Litter mass remaining refers to the loss of the dry weight of litter after a period of decomposition, denoted as R (%), and the equation was as follows60:
where M0 (10 g) is the initial dry weight of litter in each bag and Mt (g) is the dry weight of litter at time t. The litter decomposition rates (k) were determined by an exponential decay model61:
where M0 and Mt are the same as above. The content of organic C and N in litter was determined by potassium dichromate and sulfuric acid heating62, the Kjeldahl method63, and molybdenum antimony colorimetry64.
Statistical analysis
First, a linear mixed effects model (LMM) was performed to evaluate the effect of forest type, stand age, time and their interactions on leaf litter mass remaining, C and N loss with forest type, stand age and sample time as fixed factors and plot as a random-effect factor using the package lme4 in R v.4.2.0 (R Development Core Team, 2022). Nonmetric multidimensional scaling (NMDS) based on Bray‒Curtis distance was performed to assess the multivariate analysis of the microbial community composition of litter for the Chinese fir and naturally occurring secondary forest stands.
Second, another linear mixed effects model (LMM) was carried out to identify the main drivers of the litter decomposition rate and nutrient loss of forest ecosystems by examining biophysical parameters previously shown to influence decomposition processes. The initial model included stand age, soil environment, litter quality, microbial community and enzyme activity parameters with plot as a random effect factor. To prevent overfitting, we deleted the variable with a variance expansion factor (VIF) > 5 on the premise that the stand age was selected into the model. Afterwards, the optimal model was selected by using the ‘dredging’ function of the MuMIn package according to the Akaike information criterion (AIC) fitted with maximum likelihood.
Finally, structural equation modelling (SEM) was performed to test the effect of stand age, soil environment, litter quality, microbial community and enzyme activities on litter C and N loss by using the package lavaan in R. Soil environment (including pH, DON, TC, TN, etc.), litter quality (including C, N, lignin, cellulose, etc.), microbial community (including GN, fungi, AMF, etc.) and enzyme activity (including CBH, BG, NAG, AP, etc.) were included in the model as composite variables. We proposed a priori SEM model to evaluate the direct and indirect effects of stand age and the above-mentioned composite variables on litter C and N loss. We retained the soil environment (SM or ) and enzyme activity (NAG or PEO) indicators in the final SEM models, which had significant effect on litter quality, microbial community, and enzyme activities. We conducted hypothetical causal models that included all possible paths and improved the model fitting by modifying parameters (Supplementary Fig. 1). The final model was selected according to goodness-of-fit statistics and the Akaike information criterion (AIC) among several models65. We used the chi-square test (P > 0.05), GFI (GFI > 0.95) and RMSEA (RMSEA < 0.05) to indicate a good fit of the structural equation models. All statistical analyses and graphs in this article were conducted using R software v.4.2.0 (R Development Core Team, 2022).
Supplementary Information
Acknowledgements
We thank Yu Zhang, Mengjuan Wang, Songsong Ran, Gaochao Zheng and Zhenyu Wang for their valuable efforts in establishing the research plots and helping in soil sampling. Funding for this experiment was provided by the National Natural Science Foundation of China (Grant No. 32271842).
Author contributions
S.L. participated in field and laboratory work, collected and analysis data, and wrote the main manuscript text. Z.X. participated in field and laboratory work, collected and analysis data and Z.Y. designed experiments and participated in field work and Y.F. provide support for data analysis and X.S. participated in field work and B.Z. and S.W. provide support for field work and Z.H. and X.W. designed experiments, reviewed articles and provided financial support. All authors reviewed the manuscript.
Data availability
The datasets analysed during the current study are listed in Supplementary Materials. All datasets generated during the current study are available immediately after its publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-44042-5.
References
- 1.Parton W, et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science. 2007;315:361–364. doi: 10.1126/science.1134853. [DOI] [PubMed] [Google Scholar]
- 2.Cotrufo MF, et al. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015;8:776–779. doi: 10.1038/ngeo2520. [DOI] [Google Scholar]
- 3.Craig ME, et al. Fast-decaying plant litter enhances soil carbon in temperate forests but not through microbial physiological traits. Nat. Commun. 2022;13:1229. doi: 10.1038/s41467-022-28715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creamer CA, et al. Microbial community structure mediates response of soil C decomposition to litter addition and warming. Soil Biol. Biochem. 2015;80:175–188. doi: 10.1016/j.soilbio.2014.10.008. [DOI] [Google Scholar]
- 5.Bravo-Oviedo A, Ruiz-Peinado R, Onrubia R, del Río M. Thinning alters the early-decomposition rate and nutrient immobilization-release pattern of foliar litter in Mediterranean oak-pine mixed stands. For. Ecol. Manag. 2017;391:309–320. doi: 10.1016/j.foreco.2017.02.032. [DOI] [Google Scholar]
- 6.Powers JS, et al. Decomposition in tropical forests: A pan-tropical study of the effects of litter type, litter placement and mesofaunal exclusion across a precipitation gradient. J. Ecol. 2009;97:801–811. doi: 10.1111/j.1365-2745.2009.01515.x. [DOI] [Google Scholar]
- 7.Garcia-Palacios P, Maestre FT, Kattge J, Wall DH. Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol. Lett. 2013;16:1045–1053. doi: 10.1111/ele.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford MA, et al. Understanding the dominant controls on litter decomposition. J. Ecol. 2016;104:229–238. doi: 10.1111/1365-2745.12507. [DOI] [Google Scholar]
- 9.Bai X, et al. Extracellular enzyme activity and stoichiometry: The effect of soil microbial element limitation during leaf litter decomposition. Ecol. Indic. 2021 doi: 10.1016/j.ecolind.2020.107200. [DOI] [Google Scholar]
- 10.Kyaschenko J, Clemmensen K, Hagenbo A, Karltun E, Lindahl BD. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME J. 2017;11:863–874. doi: 10.1038/ismej.2016.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trogisch S, He J-S, Hector A, Scherer-Lorenzen M. Impact of species diversity, stand age and environmental factors on leaf litter decomposition in subtropical forests in China. Plant Soil. 2016;400:337–350. doi: 10.1007/s11104-015-2737-5. [DOI] [Google Scholar]
- 12.Savaci G, Sariyildiz T. Effects of stand age on litter quality, decomposition rate and nutrient release of Kazdagi fir (Abies nordmanniana subsp. equi-trojani) iForest Biogeosci. For. 2020;13:396–403. doi: 10.3832/ifor3306-013. [DOI] [Google Scholar]
- 13.Sharma G, Sharma R, Sharma E. Influence of stand age on nutrient and energy release through decomposition in alder-cardamom agroforestry systems of the eastern Himalayas. Ecol. Res. 2007;23:99–106. doi: 10.1007/s11284-007-0377-9. [DOI] [Google Scholar]
- 14.Zhang K, et al. Linking litter production, quality and decomposition to vegetation succession following agricultural abandonment. Soil Biol. Biochem. 2013;57:803–813. doi: 10.1016/j.soilbio.2012.08.005. [DOI] [Google Scholar]
- 15.Błońska E, Piaszczyk W, Staszel K, Lasota J. Enzymatic activity of soils and soil organic matter stabilization as an effect of components released from the decomposition of litter. Appl. Soil Ecol. 2021 doi: 10.1016/j.apsoil.2020.103723. [DOI] [Google Scholar]
- 16.Fanin N, Moorhead D, Bertrand I. Eco-enzymatic stoichiometry and enzymatic vectors reveal differential C, N, P dynamics in decaying litter along a land-use gradient. Biogeochemistry. 2016;129:21–36. doi: 10.1007/s10533-016-0217-5. [DOI] [Google Scholar]
- 17.Lucas-Borja ME, Hedo de Santiago J, Yang Y, Shen Y, Candel-Perez D. Nutrient, metal contents and microbiological properties of litter and soil along a tree age gradient in Mediterranean forest ecosystems. Sci. Total Environ. 2019;650:749–758. doi: 10.1016/j.scitotenv.2018.09.079. [DOI] [PubMed] [Google Scholar]
- 18.Smith AP, Marín-Spiotta E, Balser T. Successional and seasonal variations in soil and litter microbial community structure and function during tropical postagricultural forest regeneration: A multiyear study. Glob. Change Biol. 2015;21:3532–3547. doi: 10.1111/gcb.12947. [DOI] [PubMed] [Google Scholar]
- 19.Carney KM, Matson PA. The influence of tropical plant diversity and composition on soil microbial communities. Microb. Ecol. 2006;52:226–238. doi: 10.1007/s00248-006-9115-z. [DOI] [PubMed] [Google Scholar]
- 20.Lebrija-Trejos E, Pérez-García EA, Meave JA, Poorter L, Bongers F. Environmental changes during secondary succession in a tropical dry forest in Mexico. J. Trop. Ecol. 2011;27:477–489. doi: 10.1017/s0266467411000253. [DOI] [Google Scholar]
- 21.NFG. Overview of China’s forest Resources-The ninth National Forest resources inventory. (ed. NFG) 8–19 (2019).
- 22.Jiang Y, et al. Growth characteristics of Cunninghamia lanceolata in China. Sci. Rep. 2022;12:18179. doi: 10.1038/s41598-022-22809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu XJ, Fan RX, Chen Z, Wang QF, Yu GR. Eddy covariance-based differences in net ecosystem productivity values and spatial patterns between naturally regenerating forests and planted forests in China. Sci. Rep. 2022;12:20556. doi: 10.1038/s41598-022-25025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan X, et al. Functional identity drives tree species richness-induced increases in litterfall production and forest floor mass in young tree communities. New Phytol. 2023 doi: 10.1111/nph.19216. [DOI] [PubMed] [Google Scholar]
- 25.Jing H, et al. Effect of nitrogen addition on the decomposition and release of compounds from fine roots with different diameters: The importance of initial substrate chemistry. Plant Soil. 2019;438:281–296. doi: 10.1007/s11104-019-04017-w. [DOI] [Google Scholar]
- 26.Purahong W, et al. Influence of different forest system management practices on leaf litter decomposition rates, nutrient dynamics and the activity of ligninolytic enzymes: A case study from Central European Forests. PLoS One. 2014 doi: 10.1371/journal.pone.0093700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai A, et al. Patterns and driving factors of litter decomposition across Chinese terrestrial ecosystems. J. Clean. Prod. 2021 doi: 10.1016/j.jclepro.2020.123964. [DOI] [Google Scholar]
- 28.Lidman J, Jonsson M, Burrows RM, Bundschuh M, Sponseller RA. Composition of riparian litter input regulates organic matter decomposition: Implications for headwater stream functioning in a managed forest landscape. Ecol. Evol. 2017;7:1068–1077. doi: 10.1002/ece3.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hobbie SE, et al. Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology. 2006;87:2288–2297. doi: 10.1890/0012-9658(2006)87[2288:TSEODA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Ochoa-Hueso R, et al. Ecosystem type and resource quality are more important than global change drivers in regulating early stages of litter decomposition. Soil Biol. Biochem. 2019;129:144–152. doi: 10.1016/j.soilbio.2018.11.009. [DOI] [Google Scholar]
- 31.Su X, et al. Understory vegetation dynamics of Chinese fir plantations and natural secondary forests in subtropical China. For. Ecol. Manag. 2021 doi: 10.1016/j.foreco.2020.118750. [DOI] [Google Scholar]
- 32.Singhal V, Roy T, Singh C, Ghosh J. Effect of incubation time, litter diversity and species richness on decomposition dynamics of tree species from western Himalayas. Catena. 2021 doi: 10.1016/j.catena.2021.105281. [DOI] [Google Scholar]
- 33.Lin H, et al. What drives leaf litter decomposition and the decomposer community in subtropical forests—The richness of the above-ground tree community or that of the leaf litter? Soil Biol. Biochem. 2021 doi: 10.1016/j.soilbio.2021.108314. [DOI] [Google Scholar]
- 34.Handa IT, et al. Consequences of biodiversity loss for litter decomposition across biomes. Nature. 2014;509:218–221. doi: 10.1038/nature13247. [DOI] [PubMed] [Google Scholar]
- 35.Liao C, Long C, Zhang Q, Cheng X. Stronger effect of litter quality than micro-organisms on leaf and root litter C and N loss at different decomposition stages following a subtropical land use change. Funct. Ecol. 2022;36:896–907. doi: 10.1111/1365-2435.13999. [DOI] [Google Scholar]
- 36.Yang K, et al. Litter decomposition and nutrient release from monospecific and mixed litters: Comparisons of litter quality, fauna and decomposition site effects. J. Ecol. 2022;110:1673–1686. doi: 10.1111/1365-2745.13902. [DOI] [Google Scholar]
- 37.Zhang D, Hui D, Luo Y, Zhou G. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008;1:85–93. doi: 10.1093/jpe/rtn002. [DOI] [Google Scholar]
- 38.Day T, Guénon R, Ruhland C. Photodegradation of plant litter in the Sonoran Desert varies by litter type and age. Soil Biol. Biochem. 2015;89:109–122. doi: 10.1016/j.soilbio.2015.06.029. [DOI] [Google Scholar]
- 39.Day TA, Urbine JM, Bliss MS. Supplemental precipitation accelerates decay but only in photodegraded litter and implications that sunlight promotes leaching loss. Biogeochemistry. 2022;158:113–129. doi: 10.1007/s10533-022-00888-x. [DOI] [Google Scholar]
- 40.De Long JR, et al. Understory plant functional groups and litter species identity are stronger drivers of litter decomposition than warming along a boreal forest post-fire successional gradient. Soil Biol. Biochem. 2016;98:159–170. doi: 10.1016/j.soilbio.2016.04.009. [DOI] [Google Scholar]
- 41.Chen Y, et al. Stand age and species traits alter the effects of understory removal on litter decomposition and nutrient dynamics in subtropical Eucalyptus plantations. Glob. Ecol. Conserv. 2019 doi: 10.1016/j.gecco.2019.e00693. [DOI] [Google Scholar]
- 42.Petraglia A, et al. Litter decomposition: Effects of temperature driven by soil moisture and vegetation type. Plant Soil. 2018;435:187–200. doi: 10.1007/s11104-018-3889-x. [DOI] [Google Scholar]
- 43.Liu Y, Tian H, Liu S, Li G, Hu X. Asymmetric effects between tree and understorey litters on mixed litter decomposition in temperate Quercus variabilis forest. Sci. Total Environ. 2022;806:150939. doi: 10.1016/j.scitotenv.2021.150939. [DOI] [PubMed] [Google Scholar]
- 44.Pastorelli R, et al. Litter decomposition: Little evidence of the “home-field advantage” in a mountain forest in Italy. Soil Biol. Biochem. 2021 doi: 10.1016/j.soilbio.2021.108300. [DOI] [Google Scholar]
- 45.Krishna M, et al. Successional trajectory of bacterial communities in soil are shaped by plant-driven changes during secondary succession. Sci. Rep. 2020;10:9864. doi: 10.1038/s41598-020-66638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paterson E, et al. Labile and recalcitrant plant fractions are utilised by distinct microbial communities in soil: Independent of the presence of roots and mycorrhizal fungi. Soil Biol. Biochem. 2008;40:1103–1113. doi: 10.1016/j.soilbio.2007.12.003. [DOI] [Google Scholar]
- 47.Jing H, Wang G. Temporal dynamics of Pinus tabulaeformis litter decomposition under nitrogen addition on the Loess Plateau of China. For. Ecol. Manag. 2020 doi: 10.1016/j.foreco.2020.118465. [DOI] [Google Scholar]
- 48.Bray SR, Kitajima K, Mack MC. Temporal dynamics of microbial communities on decomposing leaf litter of 10 plant species in relation to decomposition rate. Soil Biol. Biochem. 2012;49:30–37. doi: 10.1016/j.soilbio.2012.02.009. [DOI] [Google Scholar]
- 49.Razavi BS, Blagodatskaya E, Kuzyakov Y. Temperature selects for static soil enzyme systems to maintain high catalytic efficiency. Soil Biol. Biochem. 2016;97:15–22. doi: 10.1016/j.soilbio.2016.02.018. [DOI] [Google Scholar]
- 50.Waring BG. Exploring relationships between enzyme activities and leaf litter decomposition in a wet tropical forest. Soil Biol. Biochem. 2013;64:89–95. doi: 10.1016/j.soilbio.2013.04.010. [DOI] [Google Scholar]
- 51.WRB, I.W.G. World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. (ed. WRB, I.W.G.) (2015).
- 52.Wan X, et al. Soil C: N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil. 2014;387:103–116. doi: 10.1007/s11104-014-2277-4. [DOI] [Google Scholar]
- 53.Huang Z, et al. Soil microbial biomass, community composition and soil nitrogen cycling in relation to tree species in subtropical China. Soil Biol. Biochem. 2013;62:68–75. doi: 10.1016/j.soilbio.2013.03.008. [DOI] [Google Scholar]
- 54.Frostegård Å, Tunlid A, Bååth E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011;43:1621–1625. doi: 10.1016/j.soilbio.2010.11.021. [DOI] [Google Scholar]
- 55.Denef K, Roobroeck D, Manimel Wadu MCW, Lootens P, Boeckx P. Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil Biol. Biochem. 2009;41:144–153. doi: 10.1016/j.soilbio.2008.10.008. [DOI] [Google Scholar]
- 56.Bossio DA, Scow KM. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profles and substrate utilization patterns. Microb. Ecol. 1998;35:265–278. doi: 10.1007/s002489900082. [DOI] [PubMed] [Google Scholar]
- 57.Frostegård Å, Baath E, Tunlid A. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty-acid analysis. Soil Biol. Biochem. 1993;25:723–730. doi: 10.1016/0038-0717(93)90113-P. [DOI] [Google Scholar]
- 58.Ruess L, Chamberlain PM. The fat that matters: Soil food web analysis using fatty acids and their carbon stable isotope signature. Soil Biol. Biochem. 2010;42:1898–1910. doi: 10.1016/j.soilbio.2010.07.020. [DOI] [Google Scholar]
- 59.German DP, et al. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011;43:1387–1397. doi: 10.1016/j.soilbio.2011.03.017. [DOI] [Google Scholar]
- 60.Wang F, et al. Meiofauna promotes litter decomposition in stream ecosystems depending on leaf species. Ecol. Evol. 2020;10:9257–9270. doi: 10.1002/ece3.6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olson J. Energy storage and the balance of producers and decomposers in ecological systems. Ecology. 1963;44:322–331. doi: 10.2307/1932179. [DOI] [Google Scholar]
- 62.Mebius L. A rapid method for the determination of organic carbon in soil. Analytica Chimica Acta. 1960;22:120–124. doi: 10.1016/S0003-2670(00)88254-9. [DOI] [Google Scholar]
- 63.Page A, Miller R, Dennis R. Methods of Soil Analysis. American Society of Agronomy; 1982. [Google Scholar]
- 64.Lu RK. Methods for Soil Agrochemistry Analysis. Agricultural Science and Technology Press; 2000. [Google Scholar]
- 65.Chen X, et al. Tree diversity increases decadal forest soil carbon and nitrogen accrual. Nature. 2023 doi: 10.1038/s41586-023-05941-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are listed in Supplementary Materials. All datasets generated during the current study are available immediately after its publication.