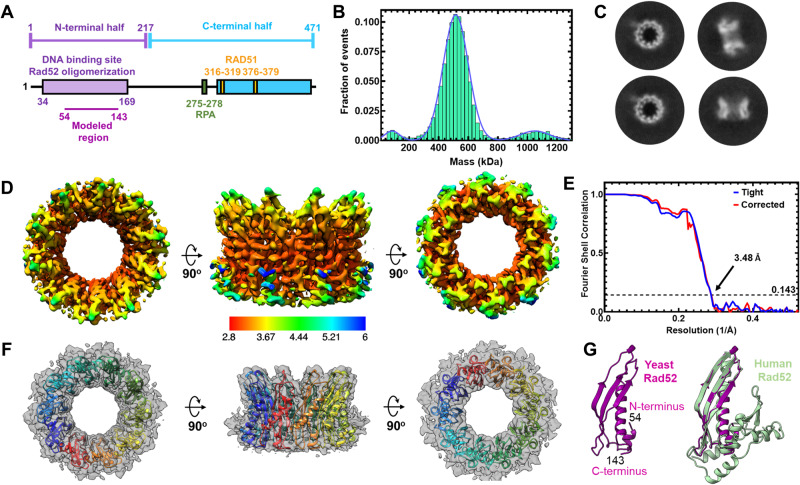
Fig. 1. S. cerevisiae Rad52 functions as a homodecamer.
A Schematic representation of a yeast Rad52 subunit depicts N- and C-terminal regions that promote DNA binding, oligomerization, & protein–protein interactions. B Mass photometry analysis predominantly shows a single Rad52 species. The major species (>85%) shows a mass of 498.4 ± 23.8 kDa. A minor 991.8 ± 75.34 kDa species (~8–12%) is observed along with a small fraction of free monomers (72.9 ± 13.9 kDa). C Representative 2D classes of full-length yeast Rad52 observed in cryo-EM analysis and used for determination of the structure. D Color coded depiction of the local resolution mapped on to the Rad52 cryo-EM map. E Gold Standard Fourier shell correlation (FSC) curves estimated using Cryosparc. F De novo built atomic model of Rad52 overlaid on the cryo-EM map. G Atomic model of a Rad52 subunit (magenta) aligned with the crystal structure of human RAD52 (gray; PDB 1KN0) using Chimera with root-mean-square deviation (RMSD) of 0.849 Å for Cα atoms of 88 amino acid pairs. Source data are provided as a Source data file.