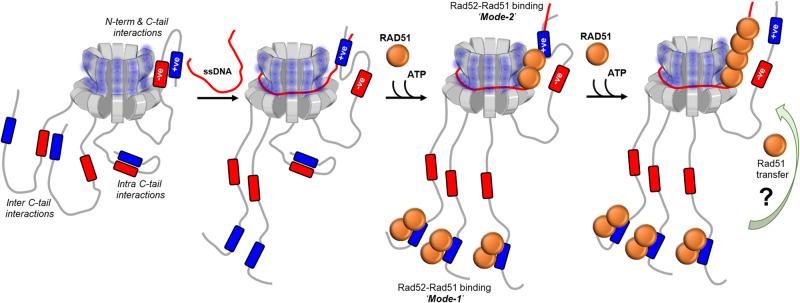
Fig. 8. Model describing the mechanism of action of Rad52 and regulation by the C-tail.
The homodecameric ring structure of the N-terminal half of S. cerevisiae is depicted and four of the ten disordered C-terminal tails are shown for clarity. The negative and positive patches are shown and the asymmetric interaction between one tail and the N-terminal half is depicted. We propose that such interactions within the remaining tails are suppressed through either intra or inter C-tail interactions between the negative and positive patches. The path of ssDNA binding is modulated through interactions between the DNA backbone and the positive patch bound to the N-terminal half. The bipartite Rad51 interactions are depicted. Mode-1 and Mode-2 are Rad51 interactions with the C- and N-terminal regions, respectively. The binding and remodeling of Rad51 in the presence of DNA and the potential redistribution of Rad51 molecules is speculative. Higher resolution structures will be required to better define the details of the interaction between the two proteins. We propose that the asymmetric interactions promote single-position nucleation and growth of pre-synaptic Rad51 filaments in HR.