Figure 4. Female mice have an age-dependent neuronal requirement for glycolysis.
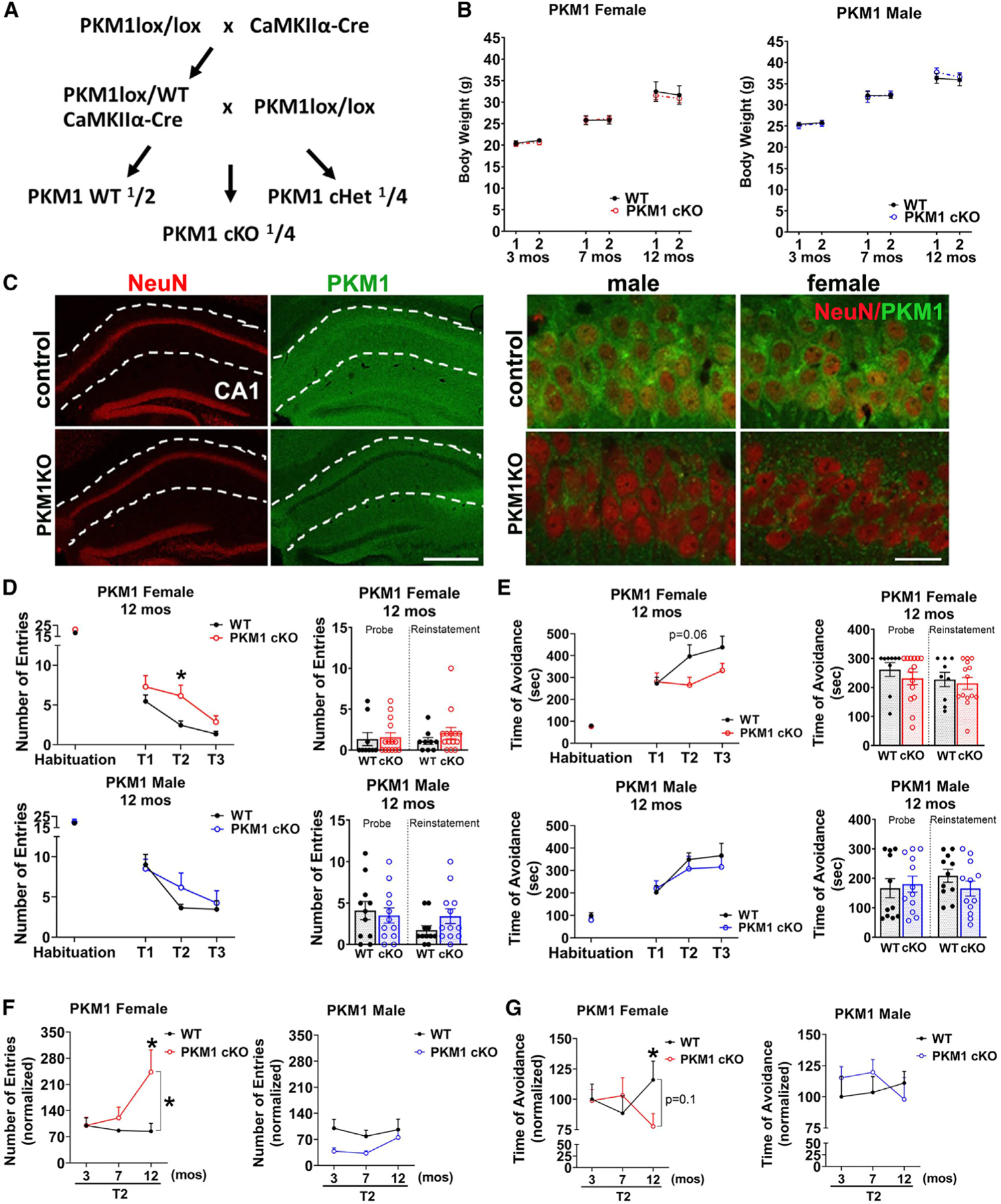
(A) Breeding scheme to generate mice with conditional postnatal deletion of PKM1 in CA1 and other forebrain neurons.
(B) PKM1cKO mice have similar weights to controls. Repeated weight measurements at 3, 7, and 12 months of age. Data are means ± SEM; n = 10–11 PKM1 WT and 14 KO females, and 11 or 12 WT and 13 KO males at each time point.
(C) PKM1 immunofluorescence shows loss of PKM in CA1 neurons in PKM1cKO mice. Sections from 12-month-old mice are stained with NeuN (red) and PKM1 (green). Scale bar, 400 mm (left), 40 mm (right).
(D–G) Female PKM1cKO mice develop age-dependent spatial learning and memory deficits as shown by active place avoidance.
(D and E) Female PKM1cKO mice have increased entrances into the aversive zone (D), and a trend of decreased maximal time of avoidance of this zone (E) at 12 months of age (p = 0.06). No deficits were observed in males.
(F and G) Longitudinal analysis shows change in second time point (T2) of active place avoidance testing, with each mouse normalized to the mean control value at 3 months. PKM1cKO females are equivalent to controls at 3 and 7 months of age, but 12-month-old PKM1cKO mice enter the aversive zone more frequently than controls (F), and avoid it for less time (G), whereas no deficits were observed in males (see Figure S4 for full data from 3 to 7 months). n = 10–11 WT, 14 KO females, and 11 or 12 WT and 13 KO males, each compiled from three cohorts.
*p % 0.05 by Welch ANOVA with Dunnett’s T3 multiple comparisons test (D and E) and Welch’s t tests (F and G). Brackets in graphs (F and G) show significance of linear mixed modeling for the interaction of genotype and age (F and G).
