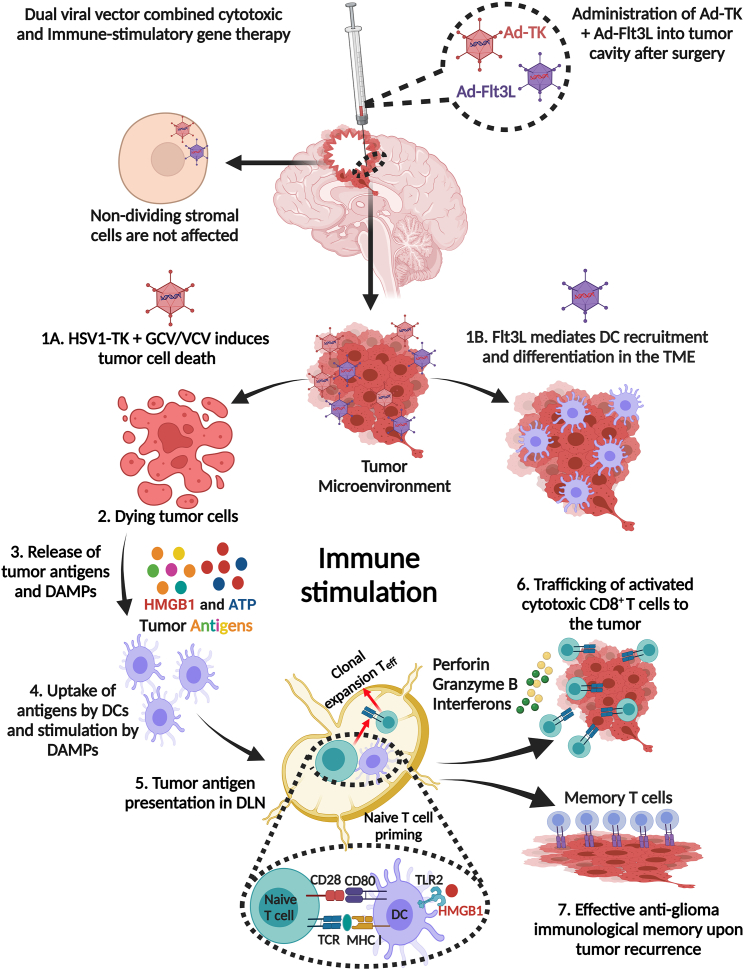
Figure 1.
Mechanistic details of the dual viral-vector combined cytotoxic and immune-stimulatory gene therapy
After surgical resection, first-generation adenoviral vectors (Ads) encoding HSV1-thymidine kinase (TK) and HSV1-FMS-like tyrosine kinase 3 ligand (Flt3L) are injected into the tumor cavity, followed by administration of the prodrug ganciclovir (GCV) (rodents), or valacyclovir (VCV) (humans). Ad-TK selectively targets dividing tumor cells without damaging non-dividing stromal cells. (1A) Ad-TK induces glioma cell death in the presence of the prodrug (VCV), which is administered systemically. (1B) Flt3L promotes recruitment and differentiation of DCs in the glioma TME. Tumor cells infected with Ad-Flt3L express Flt3L, which is released into the circulation, inducing DC expansion, migration, and accumulation in the TME from the bone marrow. (2) Tumor cells infected with Ad-TK express TK protein that phosphorylates GCV to GCV/VCV-monophosphate (GCVp/VCVp), which is further phosphorylated by cellular kinases to the tri-phosphorylated form (GCVp3/VCVp3; purine analog), which selectively inhibits DNA synthesis, leading to DNA breaks and apoptosis in proliferating glioma cells. Phosphorylated GCV/VCV is passively transported to surrounding TK-non-expressing cells via gap junction intercellular communication inducing cell death. This bystander effect enhances the antitumor effect of Ad-TK gene therapy. (3) The expression of TK in the presence of GCV/VCV also leads to the release of glioma-specific antigens and DAMPs, such as HMBG1, calreticulin, and ATP from dying glioma cells. (4) DCs are recruited into the TME by Flt3L and take up tumor antigens released from the dying glioma cells, with DAMPs further stimulating immune responses. These DAMPs bind their corresponding receptors expressed on DCs, where HMGB1 binds to TLR2 to promote cytokine production and tumor antigen cross-presentation, extracellular ATP binds to the purinergic receptor P2X7R to promote DC recruitment, while calreticulin binds to the CD91 receptor involved in immunosurveillance. (5) DCs loaded with glioma-specific antigens migrate to the cervical draining lymph node (DLN) where they present the antigens to naive T cells on MHC I, resulting in the priming and clonal expansion of glioma antigen-specific effector T cells (Teff) with anti-glioma immunity. (6) Primed CD8+ effector T cells enter the circulation from the DLN to the TME and kill glioma cells through the production of granzyme B, perforin, and effector cytokine IFN-ɣ. (7) Continued exposure of T cells to glioma antigens promotes immunological memory, resulting in the inhibition of tumor recurrence through the presence of memory T cells (CD103 and CD69) that facilitate the ongoing anti-glioma immune response especially as the tumor recurs. The figure was created with BioRender.com.