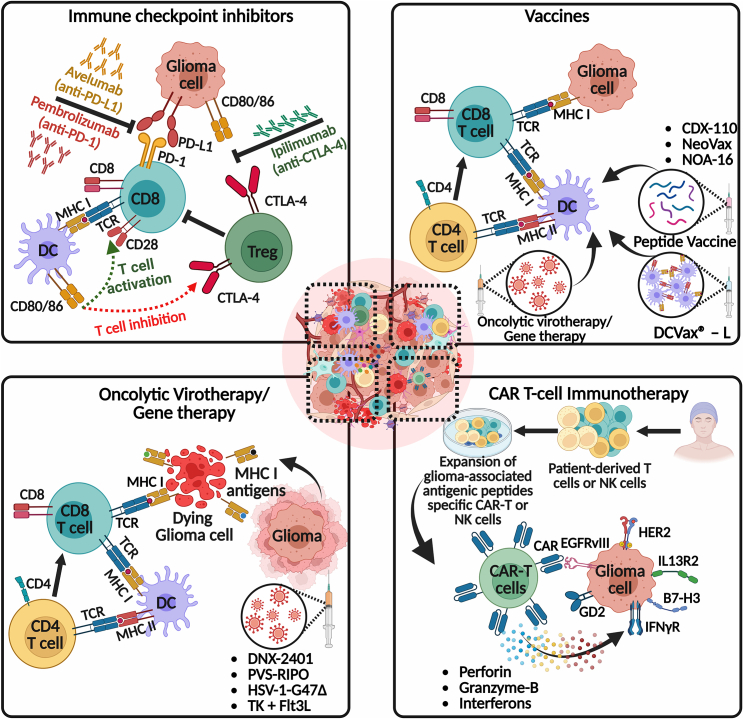
Figure 3.
Potential combination strategies for inducing anti-glioma immune responses
This figure explores the potential of immune checkpoint inhibitors, DC vaccines, immunovirotherapy, and CAR-T cells, and their implications for future clinical trials when combined with our TK + Flt3L gene therapy. The top left panel shows the immune checkpoint inhibitors, such as pembrolizumab (anti-PD-1), avelumab (anti-PD-L1), and ipilimumab (anti-CTLA-4), which aim to combat T cell exhaustion or immunosuppression by blocking immune checkpoints, thereby restoring T cell functionality and enhancing anti-glioma immunity. The top right panel shows the activation and recruitment of tumor neo-antigen-specific T cells into the TME to selectively target and kill glioma cells in response to vaccination. The current state-of-the-art vaccination approaches educate T cells to target glioma neo-antigens, such as injection of ex-vivo-developed matured DCs loaded with either autologous tumor cell lysate (DC vaccines; DCVax-L) or peptides, injection of synthetic peptides plus immune adjuvant (peptide vaccines; CDX-110, NeoVax, and NOA-16) and intratumoral oncolytic virotherapy (localized vaccination). In the bottom left panel, various oncolytic virotherapy strategies, including DNX-2401, PVS-RIPO, and HSV-1-G47Δ, are depicted. These treatments enhance the recruitment and functionality of immune cells, shift primed CD8+ immune cells toward an anti-glioma phenotype, and thereby reduce immune suppression within the TME. The activation of anti-glioma immunity by oncolytic virotherapy/gene therapy often leads to the production of various pro-inflammatory cytokines, which further help to generate an immune-stimulatory hot TME (Figures 1 and 2). The bottom right panel illustrates CAR immunotherapies, which involve genetically engineering a patient’s own T cells or non-patient NK-92 cells to express neo-antigen-specific CARs. These CARs are expanded in culture and then transferred to the patient adoptively. Once infused into the patient, the CAR-T cells recognize tumor-specific antigenic peptides and execute effector functions, such as releasing antitumor cytokines (perforin, granzyme-B, and interferons), leading to the eradication of target cells. Several glioma-specific cell surface antigens are being investigated for their potential as targets for CAR-T cell therapy. These antigens include IL13R2, EGFR/EGFRvIII, HER2, disialoganglioside GD2, and B7-H3. Promising results have been reported in preclinical research and clinical trials. The figure was created with BioRender.com.