Figure 1.
Generation and validation of DARPin-based hFAP-specific adenoviral retargeting adapters
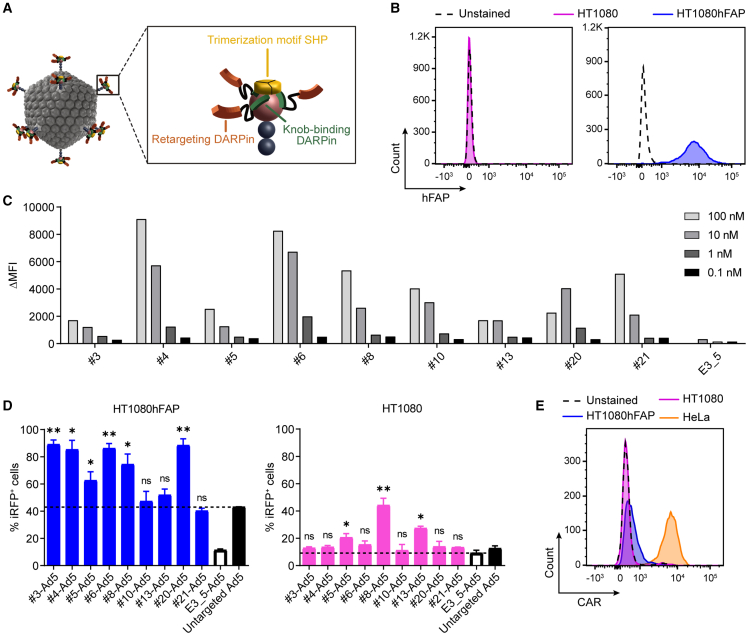
(A) Schematic representation of a bispecific trimeric DARPin adapter for adenoviral retargeting. The retargeting DARPin (orange) with specificity for a selected cell surface molecule (e.g., FAP) is fused via a long flexible linker to the knob-binding DARPin 1D3 (green) that in turn is fused via a short linker to the trimerizing protein SHP from lambdoid phage (yellow). The bispecific trimeric DARPin adapter forms a highly stable clamp around the fiber knob (red) to block natural cellular interactions and redirect adenoviral tropism to selected cells (e.g., FAP+ cells). (B) Flow cytometry analysis of hFAP expression of the parental HT1080 and HT1080hFAP cell line upon hFAP antibody staining. (C) Cell-based adapter binding assay on target and non-target cells. The purified “Top 9” adapters constructed with the selected hFAP-specific DARPins were titrated on hFAP+ HT1080hFAP and hFAP– HT1080 cells in the concentration range of 0.1–100 nM. Binding was detected via flow cytometry by staining of the His-tagged adapter, and specific binding signals were determined as ΔMFI = MFI (HT1080hFAP cells) − MFI (HT1080 cells). The non-binding control adapter E3_5 was applied as a negative binding control. Bars represent specific binding signals of single point measurements. Representative data of two independent experiments are shown. MFI, mean fluorescent intensity. (D) Transduction of target and non-target cells by hFAP adapter-retargeted Ad5. Recombinant Ad5 encoding iRFP670 was pre-incubated with the “Top 9” hFAP adapters (colored filled bars) or the E3_5 blocking adapter (black empty bar) and tested for transduction of hFAP+ HT1080hFAP and hFAP– HT1080 cells in comparison with the untargeted Ad5 (black filled bar) at a multiplicity of infection (MOI) of 20 plaque-forming units (PFU)/cell. Transduction was assessed from cellular expression of iRFP670 detected by flow cytometry. Dashed lines indicate cut-off levels above which functional and hFAP-specific adapters were identified. Bars represent mean transduction level of two biological replicates ± standard deviation (SD). Statistics: unpaired t test; ∗p < 0.05, ∗∗p < 0.005; p values are indicated for each sample with respect to the untargeted Ad5 for HT1080hFAP cells or with respect to the E3_5-Ad5 for HT1080 cells. Representative data of three independent experiments are shown. (E) Flow cytometry analysis for CAR expression of the HT1080 and HT1080hFAP cell line in comparison with the positive control HeLa cell line upon CAR antibody staining.