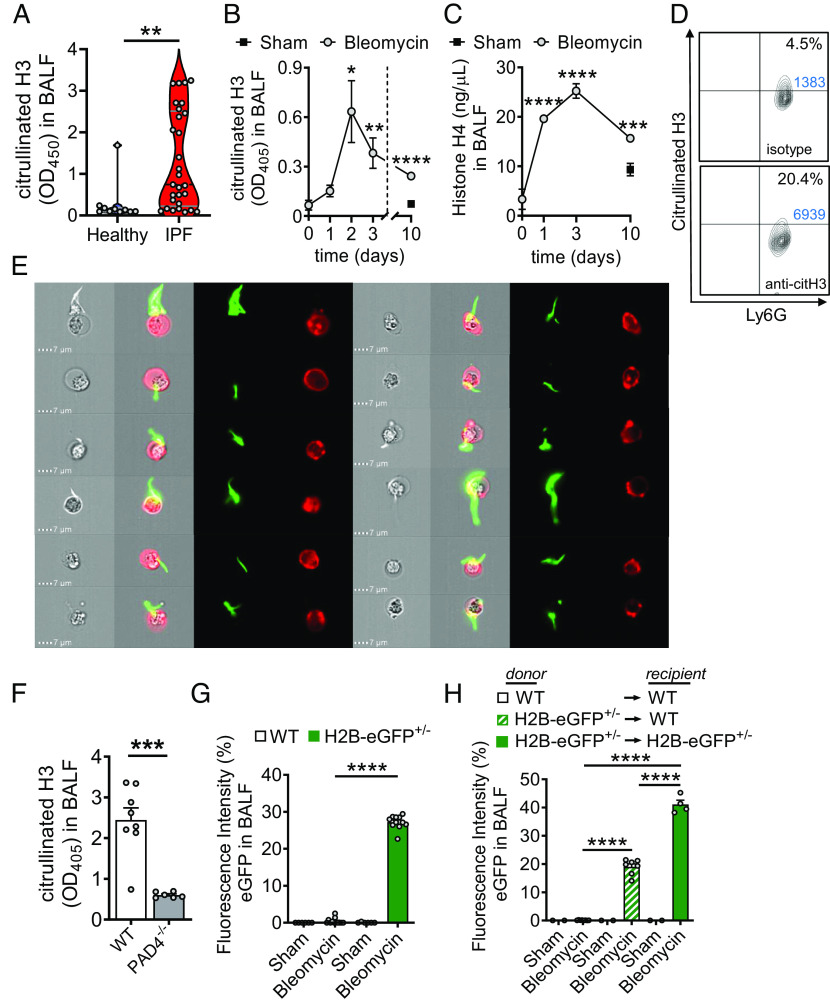
Fig. 1.
Cellular sources of externalized histones during pulmonary fibrosis. (A) Presence of externalized citrullinated histone H3 in BALF samples of healthy human controls (n = 10) and patients with IPF (n = 29), ELISA with optical density (OD) measurements at 405 nm. (B and C) Time course of citrullinated histone H3 (B) and Histone H4 (C) in cell-free BALF of C57BL/6 J mice (n ≥ 4/group for each time point) after treatment with bleomycin i.t. or NaCl 0.9% i.t. (sham; negative control), ELISA. (D) NET formation detected as externalized citrullinated histone H3 on nonpermeabilized Ly6G+CD11b+ neutrophils in BALF of C57BL/6 J wild-type (WT) mice 2 d after bleomycin i.t. (n = 6 mice/group), flow cytometry with pregating on CD11b. Absolute cell numbers in the quadrants of interest are shown in blue font. (E) Visualization of NETs from BALF cells, which were obtained 2 d after bleomycin i.t. and stained for citrullinated externalized histone H3 (green) together with Ly6G (red) for neutrophils, ImageStreamX Mark II imaging flow cytometry. (F) PAD4−/− mice and WT mice were subjected to bleomycin-induced lung injury and citrullinated H3 was detected in cell-free BALF after 2 d. (G) Detection of green fluorescence in cell-free BALF of histone H2B-eGFP fusion protein reporter mice (H2B-eGFP+/−) 2 d after bleomycin administration as compared to WT control mice. Fluorescence of a FITC-labeled control antibody was used for normalization (=100% value). (H) Chimeric mice were generated by transplantation of H2B-eGFP+/− donor bone marrow into irradiated WT recipients. In the control groups, syngeneic bone marrow was transplanted. After 5 wk, all mice received bleomycin i.t. and H2B-eGFP fluorescence was detected in cell-free BALF another 2 d later. Numbers of mice in frames F–H are indicated by circles; A: Mann–Whitney test, B and C: one-way ANOVA, F–H: Student’s t test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.