Abstract
Background:
Sequential triple combination therapy is recommended for pulmonary arterial hypertension (PAH) patients who are not at therapeutic goal on dual therapy, but long-term data on efficacy and safety is scarce.
Objective:
To assess the long-term impact of sequential triple combination therapy in patients with PAH who are not at goal on dual combination therapy.
Study Design and Methods:
We performed a retrospective observational study in a racially/ethnically diverse cohort of consecutive PAH patients on a stable dual therapy regimen who remained in intermediate- or high-risk category and were subsequently initiated on sequential triple combination therapy. We studied interval change in functional, echocardiographic, and hemodynamic parameters, REVEAL 2.0 risk category and ERS/ESC 2022 simplified four-strata risk category. Multivariate logistic regression analysis was performed to identify independent predictors of successful risk reduction (achievement or maintenance of REVEAL 2.0 low-risk category). Kaplan–Meier survival curves were created to assess the effect of risk reduction on survival.
Results:
Out of 414 PAH patients seen in our program, 55 patients received add-on sequential triple combination regimen and had follow-up hemodynamic data. The mean age was 57 years, with 85% women. The most common etiology of PAH was idiopathic/heritable (41.8%). Most patients were WHO functional class III (76.4%), and 34.5% of patients were in high-risk category (REVEAL 2.0). On a median follow-up of 68 weeks, there was a significant improvement in WHO Functional Class (p < 0.001), six-minute walk distance (35 m) with 61.8% of patients achieving low-risk status by REVEAL 2.0, and a 28% of patients’ improvement in pulmonary vascular resistance. Female gender was identified as a strong predictor of successful risk reduction, whereas Hispanic ethnicity estimated right atrial pressure on echocardiogram and pericardial effusion predicted lower probability of risk reduction. Patients who achieved or maintained low-risk status had significantly improved survival.
Conclusion:
Add-on sequential triple combination therapy significantly increased functional, echocardiographic, and hemodynamic parameters with improvement in risk category and survival.
Keywords: add-on sequential combination therapy, four-strata risk category, prostacyclin analogs, pulmonary arterial hypertension, REVEAL 2.0, and risk-stratification in PAH, triple combination therapy
Introduction
Treatment landscape for pulmonary arterial hypertension (PAH) has evolved over the past decade leading to significant improvement in survival and functional outcomes. The REVEAL registry (Registry to Evaluate Early and Long-Term PAH Disease Management) demonstrated 1-, 3-, 5-, and 7-year survival rates of 85%, 68%, 57%, and 49%, respectively. 1 Median long-term survival has increased from 2.8 years in the 1980s, reflecting the natural course of the disease, to 7 years in the modern era when therapeutic modalities became available. 2 Furthermore, risk stratifying patients into low-, intermediate-, or high-risk categories is essential in prognostication and devising treatment strategies. 3 There are several predictive risk-stratification models derived from the European and U.S REVEAL registries.4,5 Currently, the goal of pharmacotherapy in PAH is to improve functional status, right ventricular function, hemodynamics, achieve low-risk status, and delay disease progression. There is paucity of literature on identifying true responders to therapy or those who will achieve significant risk reduction with add-on sequential therapy as it may assist in determining need for escalating therapy versus early referral for lung transplantation.
Dual combination therapies in PAH is the standard of care for patients with low- and intermediate-risk status based on several clinical trials, most notably the AMBITION trial. 6 In patients at intermediate-low risk at follow-up, current guidelines recommend adding a third drug sequentially (prostacyclin receptor agonist class IIa recommendation) or switching phosphodiesterase-5 (PDE-5) inhibitor to soluble guanylate stimulator (sGCs) (class IIb recommendation), while for patients at intermediate-high or high risk, it is recommended to add parenteral prostacyclin and/or evaluate for transplantation (class IIa recommendation). 7 Several trials have included subcohorts on background combination therapy who received a sequential third investigational drug, but no current trial has focused on the long-term impact of sequential triple combination therapy in patients who are intermediate- or high-risk at follow-up. While most trials have not included risk reduction as a primary endpoint, it has been suggested as a valuable target that could potentially transform the current therapeutic approach to a goal-oriented approach. 8 Due to lack of racial/ethnic diversity in most trials, the benefit of combination therapy in diverse population still needs to be evaluated.
This study aimed to assess the long-term impact of add-on sequential triple combination therapy and identify independent predictors of risk reduction with triple therapy in a diverse PAH patient cohort.
Methods
Study design
We conducted a retrospective observational study at New York University (NYU) Langone Health Pulmonary Hypertension (PH) Center from January 2017 to July 2021. The study was approved by NYU Langone institutional review board (IRB number- i21-00064).
Data collection and definitions
Collected data included demographics, etiology of PAH, functional status including WHO functional class, six-minute walk distance, the diffusion lung capacity of carbon monoxide (DLCO, % predicted), brain natriuretic peptide levels, echocardiographic and hemodynamic parameters obtained on right heart catheterization (RHC), and initial dual combination and add-on sequential triple combination therapies were also recorded. The data collection timeline was at baseline on a stable background regimen and follow-up after initiating a third add-on drug.
Each patient was assigned a risk score and category based on the Registry to Evaluate Long and Short-term outcomes in PAH (REVEAL 2.0) (low risk: 1–6, intermediate risk: 7–8, and high risk: 9 or above) and simplified four-strata risk category as recommended by European Respiratory Society and European Society of Cardiology guidelines on management of PH 2022.4,7,9,10 Patients were considered not at goal if they remained in intermediate-/high-risk category or low-risk category with clinical progression. Clinical progression was defined as interval worsening or persistent symptoms, lack of improvement or deterioration in functional class, exercise capacity, echocardiographic parameters, hemodynamics, and increased hospitalization (all-cause or due to right ventricular failure). Successful risk-reduction was defined as achieving or maintaining low-risk category.
It is common in our practice to use soluble guanylate cyclase (sGC) stimulator (riociguat) early in the course of the disease, including as part of the upfront dual therapy regimen and to use in succession both therapeutic optimization maneuvers (i.e., addition of non-parenteral prostacyclin and switch PDE5 inhibitor to sCG stimulator) in patients deemed not candidates for parenteral prostacyclin therapy. 11 The choice of the add-on third therapeutic agent was determined by the risk status at follow-up: patients with lower-risk scores received either oral or inhaled prostacyclin, whereas patients with higher-risk scores were initiated on parenteral prostacyclin.
The primary endpoint of the study was to assess the impact of sequential triple combination therapy on functional/risk status, echocardiographic and hemodynamic parameters, and associated adverse effects. The secondary endpoint evaluated the impact of successful risk reduction on cumulative survival. The tertiary endpoint was to identify independent predictors of successful risk reduction. In addition, subcohort analysis was performed to determine the clinical impact of triple therapy on the non-White patient cohort.
Inclusion and exclusion criteria
Patients with age 18 years and older were included in the study. Patients initiated on triple sequential therapy but had not yet had a follow-up right heart catheterization, those lost to follow-up or had significant missing data were excluded.
Statistical analysis
Continuous variables were described as mean with standard deviation for normally distributed variables and median with interquartile range for non-normally distributed variables. Categorical variables were expressed as frequency and percentages.
Normally distributed continuous variables before and after adding a third drug were compared using paired t-test, whereas non-normally distributed variables were compared using the Wilcoxon signed-rank test. Categorical variables were compared using the McNemar test and Wilcoxon signed-rank test.
Survival analysis was performed using Kaplan–Meier survival curves and Log-Rank test in patients with successful risk reduction. The duration of follow-up was truncated to 1 July 2021, or the date of expiration (whichever was earlier). Multivariate logistic regression analysis with backward elimination was performed to identify predictors of successful risk reduction (achievement or maintenance of REVEAL 2.0 low-risk category). A p value < 0.05 was used for retention, and a p value > 0.10 for exclusion. SPSS® software version 26.0 (IBM®, Armonk, NY, USA) was used for statistical analysis. Missing data were not imputed.
Results
Clinical characteristics
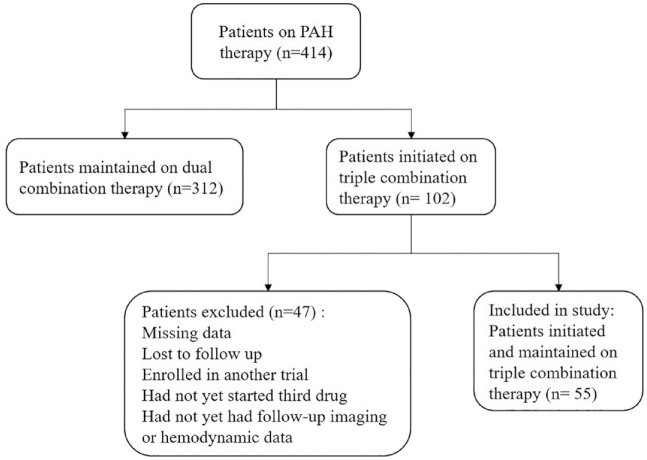
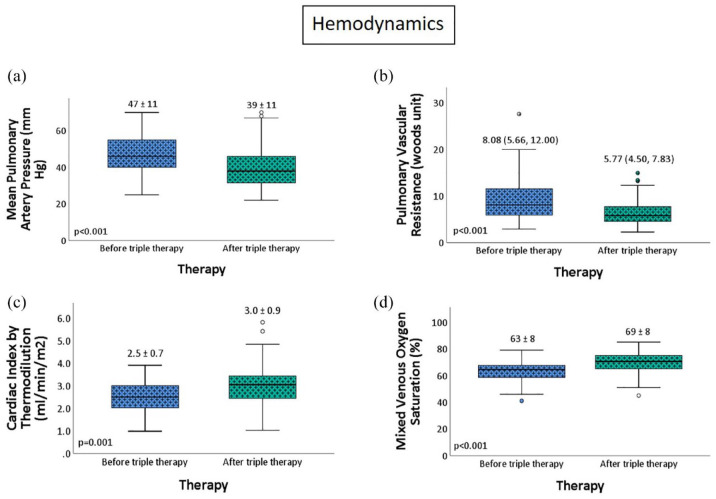
Out of 414 patients on PAH pharmacotherapy, 102 patients were initiated on triple combination therapy. Of these, 55 patients had available follow-up echocardiographic, functional, and hemodynamic data and were included in the final analysis (Figure 1). Baseline characteristics are highlighted in Table 1. The mean age of the cohort was 57 (±14) years, and there were predominantly women (85.5%). Racial/ethnical distribution of our cohort was diverse, reflective of New York City demographics, with 34.5% Black, 25.5% White, 21.8% Hispanic/Latino, and 10.9% Asian patients. The most common etiology of PAH was idiopathic/heritable (41.8%), followed by connective tissue disorder (CTD) associated with PAH (30.9%). Up to 76.4% of patients were classified under WHO FC III before initiating the third sequential drug. The mean initial six-min walk distance was 282 (±126) m. The median REVEAL 2.0 score was 7 (5, 10), with 34.5% of patients were in the high-risk category, 27.3% of them were in the intermediate-risk category, and 38.2% of them were in the low-risk category. On four-strata risk scoring, 10.9% of patients were in low-risk category, 47.4% of them were in intermediate-low-risk category, 32.7% of them were in intermediate-high-risk category, and 9.1% of them were in high-risk category. On echocardiogram, mean tricuspid regurgitant (TR) flow velocity was 4.01 (±0.71) m/s. Right ventricle (RV) was severely dilated in 25.5% of patients and moderately dilated in 34.5%. Reduced right ventricular function was observed in 64.8% of patients, with 52.2% demonstrating low tricuspid annular planar systolic excursion (TAPSE) (<1.7 cm). Pericardial effusion was present in 9.3% of patients. On dual combination therapy, right heart catheterization showed mean right atrial pressure of 9 (±4) mm Hg, mean pulmonary artery pressure of 47 (±11) mm Hg, median pulmonary artery wedge pressure of 10 (7, 13) mm Hg, median pulmonary vascular resistance (PVR) of 8.08 (5.66, 12.00) Wood units, and mean cardiac index of 2.52 (±0.72) L/min/m2. The most common background regimen was oral dual combination therapy with nitric oxide pathway drugs (PDE5 inhibitor or sGC stimulator) and endothelin receptor antagonist (ERA) (81.8%).
Figure 1.
Patients on add-on sequential triple combination therapy included in the analysis.
Table 1.
Baseline characteristics of patients initiated on sequential triple combination therapy.
| Variable (n = 55) | Mean ± standard deviation/median (interquartile range)/count (%) |
|---|---|
| Demographics | |
| Age (years) | 57 ± 14 |
| Gender (Female) | 47 (85.5%) |
| Race/ethnicity | |
| Black | 19 (34.5%) |
| White | 14 (25.5%) |
| Hispanic | 12 (21.8%) |
| Asian | 6 (10.9) |
| Native American | 1 (1.8%) |
| Unknown | 3 (5.5%) |
| Body mass index (kg/m2) | 26.6 (22.6, 30.1) |
| Etiology of pulmonary arterial hypertension | |
| Idiopathic/Heritable | 23 (41.8%) |
| Connective tissue disorder | 17 (30.9%) |
| Human immunodeficiency virus infection | 5 (9.1%) |
| Congenital heart disease | 5 (9.1%) |
| Multiple risk factors for PAH | 5 (9.1%) |
| All-cause hospitalization (1-year) | 20 (36.4%) |
| All-cause mortality | 6 (10.9%) |
| Duration to first follow-up on triple therapy (weeks) | 68 (43, 122) |
| Time from diagnosis to initiation of triple therapy (weeks) | 120 (39, 296) |
| Initial therapy | |
| ERA and prostacyclin combination | 8 (14.5%) |
| PDE-5i/sGCs and parenteral prostacyclin combination | 2 (3.6%) |
| ERA and PDE-5i/sGCs combination | 45 (81.8%) |
| Final triple combination therapy | |
| ERA with PDE-5i/sGCs and parenteral prostacyclin | 14 (25.5%) |
| ERA and PDE-5i/sGCs and oral prostacyclin | 9 (16.4%) |
| ERA and PDE-5i/sGCs and inhaled prostacyclin | 8 (14.5%) |
| ERA and PDE-5i/sGCs and prostacyclin (IP2) receptor agonist | 24 (43.6%) |
| Initial functional status | |
| World health organization functional class (WHO FC) | |
| I | 0 (0%) |
| II | 10 (18.2%) |
| III | 42 (76.4%) |
| IV | 3 (5.5%) |
| REVEAL 2.0 score | 7 (5, 10) |
| REVEAL 2.0 risk category | |
| Low | 21 (38.2%) |
| Intermediate | 15 (27.3%) |
| High | 19 (34.5%) |
| ERS/ESC 2022 simplified four-strata risk category | |
| Low-risk | 6 (10.9%) |
| Intermediate-low-risk | 26 (47.3%) |
| Intermediate-high risk | 18 (32.7%) |
| High risk | 5 (9.1%) |
| Six-min walk distance (meters) | 282 ± 126 |
| Diffusion capacity of carbon monoxide (%) | 48 ± 19 |
| Brain natriuretic peptide (pg/dL) | 45.7 (26.0, 158.0) |
| Initial echocardiographic parameters | |
| Tricuspid valve regurgitation velocity (m/s) | 4.01 ± 0.71 |
| Tricuspid annulus planar systolic excursion (low) | 24 (52.2%) |
| Right ventricular function (hypokinetic) | 35 (64.8%) |
| Right ventricular size | |
| Normal | 9 (16.7%) |
| Mildly dilated | 12 (22.2%) |
| Moderately dilated | 19 (34.5%) |
| Severely dilated | 14 (25.5%) |
| Estimated right atrial pressure | 8 (3, 15) |
| Pericardial effusion | 5 (9.3%) |
| Initial hemodynamics | |
| Mean right atrial pressure (mm Hg) | 9 ± 4 |
| Systolic pulmonary artery pressure (mm Hg) | 79 ± 19 |
| Diastolic pulmonary artery pressure (mm Hg) | 31 ± 8 |
| Mean pulmonary artery pressure (mm Hg) | 47 ± 11 |
| Pulmonary artery wedge pressure (mm Hg) | 10 (7, 13) |
| Pulmonary vascular resistance (woods unit) | 8.08 (5.66, 12.00) |
| Cardiac output (L/min) | 4.50 ± 1.38 |
| Cardiac index (L/min/m2) | 2.52 ± 0.72 |
| Mixed venous oxygen saturation (%) | 63 ± 8 |
ERA, endothelin receptor antagonist; ERS/ESC, European Respiratory Society/European Society of Cardiology; PAH, pulmonary arterial hypertension; PDE-5i, phosphodiesterase 5 inhibitor; REVEAL 2.0, Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management; sGCs, soluble guanylate cyclase stimulator.
Clinical impact of sequential triple combination therapy
The median duration of interval follow-up was 68 (43, 122) weeks, and the median time from diagnosis to initiation of sequential triple therapy was 120 (39, 296) weeks. The most common sequential triple combination therapy included nitric oxide pathway drug (PDE5 inhibitor or sGC stimulator), endothelin receptor agonist, and oral prostacyclin receptor agonist (selexipag) (43.6%), followed by parenteral prostacyclin containing regimen (25.5%). The overall mortality in our cohort was 10.9%, and all-cause hospitalization rate at 1 year of follow-up was 36.4%. Three patients eventually received lung transplantation on subsequent follow-up. Patients initiated on parenteral prostacyclin therapy had severe disease with syncope, WHO FC IV, lower BMI, more hospitalization, lower right ventricular function, and worse hemodynamics than patients initiated on non-parenteral prostacyclin (Table 2).
Table 2.
Baseline differences in patients initiated on parenteral prostacyclin versus non-parenteral prostacyclin.
| Baseline variables | Non-parenteral therapy [mean ± SD/median (IQR) or frequency/%] | Parenteral therapy [mean ± SD/median (IQR) or frequency/%] | p Value |
|---|---|---|---|
| Syncope or pre-syncope | 5 (11.9%) | 6 (46.2%) | 0.039 |
| Body mass index (kg/m2) | 27.9 (23.3, 31.1) | 23.2 (21.9, 26.3) | 0.004 |
| WHO functional class IV | 0 (0%) | 3 (23.1%) | 0.006 |
| Number of hospitalization | 0.46 ± 0.72 | 1.81 ± 1.56 | <0.001 |
| Right ventricle function (hypokinetic) | 25 (59.5%) | 10 (83.3%) | 0.039 |
| Tricuspid annulus planar systolic excursion (low) | 15 (42.9%) | 9 (81.8%) | 0.019 |
| Systolic pulmonary artery pressure (mm Hg) | 75 ± 18 | 90 ± 17 | 0.005 |
| Diastolic pulmonary artery pressure (mm Hg) | 29 ± 8 | 35 ± 8 | 0.045 |
| Mean pulmonary artery pressure (mm Hg) | 45 ± 12 | 53 ± 10 | 0.033 |
| Pulmonary vascular resistance (woods units) | 7.6 (5.5, 10.4) | 12.0 (7.6, 15.9) | 0.085 |
IQR, interquartile range.
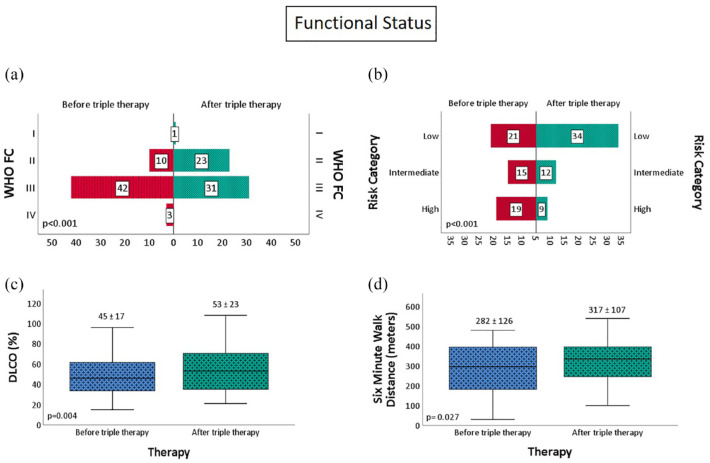
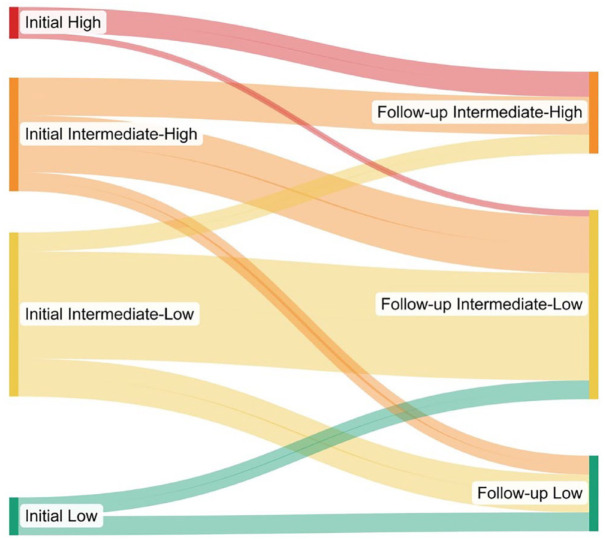
On addition of a third sequential medication, there was a significant improvement in WHO FC (p < 0.001), DLCO (17.7%, p = 0.004), 6-min walk distance (35 m, p = 0.027), and reduction in REVEAL 2.0 risk category, with up to 61.8% of patients able to achieve or maintain low-risk status (Figure 2). There was a significant improvement in four-strata risk category as well with no patient remaining in high-risk category, 21.8% (from 10.9%) of patients achieving low-risk status, 54.5% (from 47.3%) of them in intermediate-low-risk category, and 23.6% (from 32.7%) of them in intermediate-high-risk category (p = 0.001) (Figure 3).
Figure 2.
Change in (a) WHO functional class, (b) risk category, (c) diffusion capacity, and (d) six-min walk distance with sequential triple combination therapy.
Figure 3.
Sankey diagram demonstrating changes in ERS/ESC 2022 simplified four-strata risk category on addition of third sequential drug. Interestingly, no patient remained in high-risk category after addition of third sequential drug.
ERS/ESC, European Respiratory Society/European Society of Cardiology.
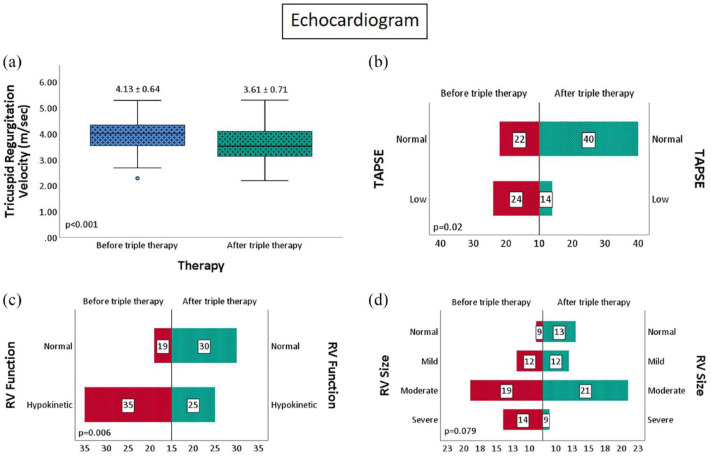
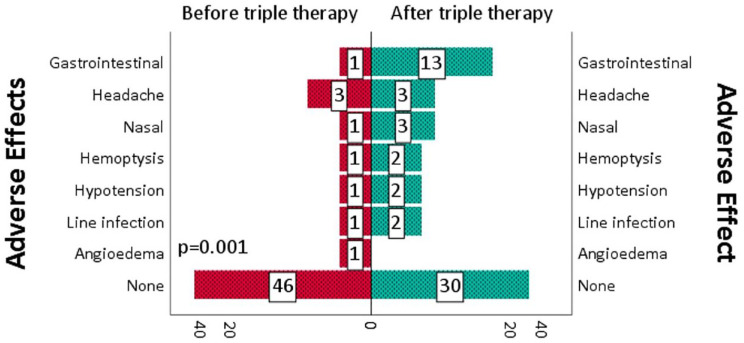
There was a significant improvement in echocardiographic parameters with a 12.5% improvement in TR velocity (p < 0.001), RV function (p = 0.006), and TAPSE (p = 0.02). (Figure 4). On follow-up right heart catheterization, there was a 17% improvement in mean pulmonary artery pressure (p < 0.001), a 28.6% improvement in PVR (p < 0.001), a 20% improvement in cardiac index (p = 0.001), and a 9% improvement in mixed venous oxygen saturation (p < 0.001) (Figure 5). Similar findings were noted in non-White patient cohort (Table 3) Interestingly, the RV size was significantly improved in the non-White cohort (p = 0.016). While most patients tolerated triple therapy well, there was a statistically significant increase in adverse effects, with gastrointestinal side effects being the most common (p = 0.001) (Figure 6).
Figure 4.
(a-d) Change in echocardiographic parameters with sequential triple combination therapy.
Figure 5.
(a-d) Change in hemodynamic parameters with sequential triple combination therapy.
Table 3.
Change in functional, echocardiographic and hemodynamic parameters in non-White cohort with sequential triple combination therapy.
| Functional status | Change in mean (Δ)/%/category (%) | p-Value |
|---|---|---|
| REVEAL 2.0 risk category (Low-risk) | 0.001 | |
| Low-risk | +9 (75%) | |
| Intermediate-risk | −1 (0.08%) | |
| High-risk | −8 (47%) | |
| 6-min walk distance (meters) | +42.6 (15.7%) | 0.025 |
| WHO functional class | 0.003 | |
| I | +1 | |
| II | +9 (180%) | |
| III | −8 (23.5%) | |
| IV | −2 (100%) | |
| Diffusion capacity of carbon monoxide (%) | +7.76 (17.5%) | 0.021 |
| Brain natriuretic peptide (pg/dL) | −4.85 (10%) | 0.376 |
| Echocardiographic parameters | ||
| Tricuspid valve regurgitation velocity (m/s) | +0.56 (13.2%) | <0.001 |
| Tricuspid annulus planar systolic excursion (Low) | +8 (40%) | 0.013 |
| Right ventricular function (hypokinetic) | +9 (30%) | 0.012 |
| Right ventricular size | 0.016 | |
| Normal | +4 (80%) | |
| Mildly dilated | 0 (0%) | |
| Moderately dilated | −3 (17.6%) | |
| Severely dilated | −3 (30%) | |
| Hemodynamics | ||
| Systolic pulmonary artery pressure (mm Hg) | −12.9 (16%) | <0.001 |
| Diastolic pulmonary artery pressure (mm Hg) | −4.1 (13.2%) | 0.007 |
| Mean pulmonary artery pressure (mm Hg) | −6.9 (14.3%) | <0.001 |
| Pulmonary artery wedge pressure (mm Hg) | −1.0 (10%) | 0.487 |
| Pulmonary vascular resistance (woods unit) | −2.61 (30.3%) | <0.001 |
| Cardiac output (L/min) | +0.62 (14.2%) | 0.011 |
| Cardiac index (L/min/m2) | +0.43 (17.6%) | 0.003 |
| Mixed venous oxygen saturation (%) | +6.6 (10.5%) | <0.001 |
Figure 6.
Adverse effects before and after initiation of sequential triple combination therapy.
Predictors of successful risk reduction
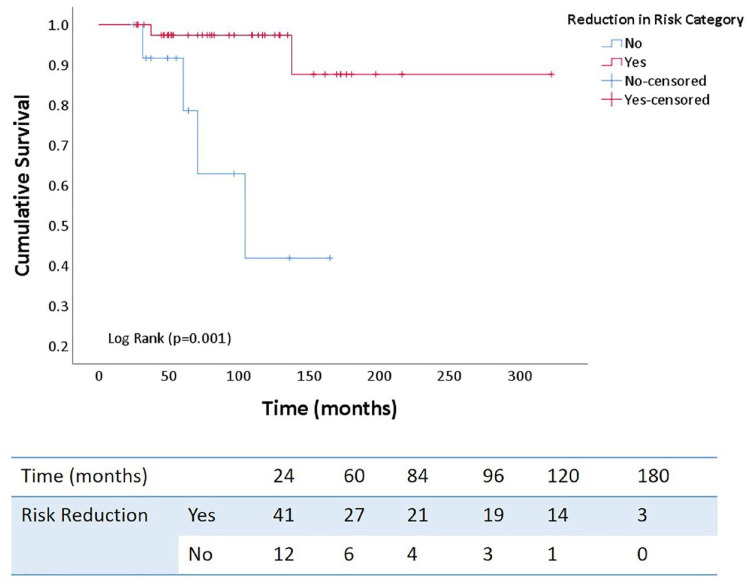
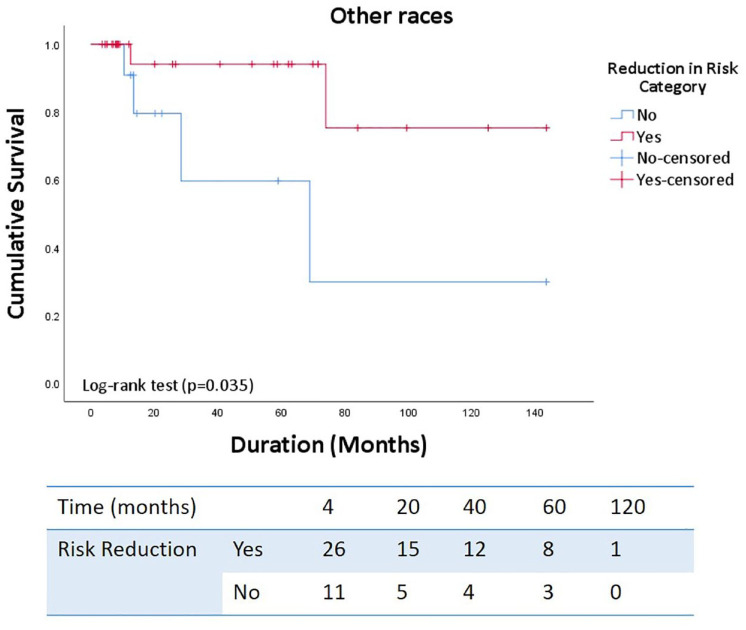
On multivariate logistic regression analysis, the female gender strongly predicted successful risk reduction. In contrast, Hispanic/Latino ethnicity, estimated right atrial pressure and presence of pericardial effusion on echocardiogram had lower odds of successful risk reduction even after excluding gender from the analysis (Tables 4 and 5). In addition, successful risk reduction was associated with significantly improved long-term survival (p = 0.001) with similar findings in non-White patient cohort. (p = 0.035) (Figures 7 and 8).
Table 4.
Univariate and multivariate logistic regression analysis to identify independent predictors of successful risk reduction.
| Variable (n = 48) | Univariate logistic regression odds ratio (95% CI, p value) | Multivariate logistic regression odds ratio (95% CI, p value) |
|---|---|---|
| Gender (female) | 8.1 (1.6–41.1, p = 0.011) | 61.2 (3.3–1150.2, p = 0.006) |
| Hispanic ethnicity | 0.19 (0.05–0.78, p = 0.021) | 0.068 (0.005–0.993, p = 0.049) |
| Connective tissue disorder | 0.23 (0.06–0.87, p = 0.030) | |
| Estimated right atrial pressure (mm Hg) | 0.83 (0.72–0.96, p = 0.013) | 0.75 (0.58–0.96, p = 0.024) |
| Tricuspid valve regurgitation velocity (m/s) | 0.99 (0.98–1.00, p = 0.090) | |
| Pericardial effusion | 0.056 (0.006–0.565, p = 0.015) | 0.013 (0.000–0.676, p = 0.031) |
| Cardiac index (L/min/m2) | 2.15 (0.86–5.36, p = 0.102) |
Table 5.
Univariate and multivariate logistic regression analysis to identify independent predictors of successful risk reduction after excluding gender from the analysis.
| Variable (n = 48) | Univariate logistic regression odds ratio (95% CI, p value) | Multivariate logistic regression odds ratio (95% CI, p value) |
|---|---|---|
| Hispanic ethnicity | 0.19 (0.05–0.78, p = 0.021) | 0.067 (0.006–0.812, p = 0.034) |
| Connective tissue disorder | 0.23 (0.06–0.87, p = 0.030) | |
| Estimated right atrial pressure (mm Hg) | 0.83 (0.72–0.96, p = 0.013) | 0.74 (0.57–0.95, p = 0.016) |
| Tricuspid valve regurgitation velocity (m/s) | 0.99 (0.98–1.00, p = 0.090) | |
| Pericardial effusion | 0.056 (0.006–0.565, p = 0.015) | 0.014 (0.000–0.657, p = 0.030) |
| Cardiac index (L/min/m2) | 2.15 (0.86–5.36, p = 0.102) | 9.6 (1.3–69.5, p = 0.025) |
Figure 7.
Kaplan–Meier survival curves demonstrating cumulative survival based on successful risk reduction with sequential triple combination therapy.
Figure 8.
Kaplan–Meier survival curves demonstrating cumulative survival in non-white cohort with successful risk reduction.
Discussion
The present study provides further evidence of significant long-term clinical benefits with add-on sequential triple therapy for patients who are not at therapeutic goal on background dual combination therapy. Risk stratification can dictate treatment choice with initial dual combination therapy for low/intermediate-risk and triple therapy with parenteral prostacyclin for intermediate/high and high-risk patients. However, up to one-third of patients may not have an appropriate hemodynamic response on follow-up, and only 34.8% of patients are able to maintain low-risk status at 6 months. 12 Kylhammar et al. 13 demonstrated long-term improvement in transplant-free survival in patients who were able to maintain low-risk status at 3-, 4-, and 5-year follow-ups in the Swedish PAH (SPAHR) registry. Interestingly, only 10–14% of patients were on triple combination therapy throughout the follow-up. Treatment becomes challenging when addressing intermediate-risk patients as they may have low-risk and high-risk features. Deciding on triple combination therapy for these patients requires further prognostication and clinical assessment of disease progression. Boucly et al. 9 found that 4-strata risk assessment (intermediate category divided into intermediate-low and intermediate-high) in the COMPERA registry had better mortality discrimination at follow-up visits, with up to 53% of patients changing the risk category compared to 39% in the 3-strata approach. As a result, the new guidelines recommend using 4-strata model on follow-up visits to guide treatment. 7 In our cohort, there was a significant improvement in 4-strata risk category, with no patient remaining in high-risk category, most patients achieving intermediate-low risk status (47.3%), and the number of patients in low-risk status doubled from 10.0% to 21.8%. However, 23.6% patients remained in intermediate-high risk category, and there were patients in whom risk category worsened despite triple drug combination, underscoring the importance of continuous follow-up and continuous need for therapeutic optimization in a sobering almost 78% of patients.
Moreover, there is a cohort of patients who demonstrate initial clinical stability with low-risk status but progress later in the disease course, thus complicating the management approach. Van de Veerdonk et al. 14 found that changes in RV function and volumes preceded clinical deterioration in patients who remained stable for the initial 5 years but had subsequent disease progression later in the course of the disease. This study highlights the importance of considering markers of disease progression as an essential variable in risk-stratifying patients and monitoring right ventricular remodeling on follow-up visits, which may otherwise not be included in risk scores.
Targeting all three known pathways and delaying pulmonary vascular and right heart remodeling has led to a more intensive approach with upfront triple combination therapy in intermediate- and high-risk patients. D’Alto et al. 15 demonstrated a reversal of right heart remodeling and a significant reduction in risk status and hemodynamics in patients initiated on upfront triple combination therapy with subcutaneous treprostinil. Boucly et al. 16 demonstrated a 91% survival rate at 5 years in patients who started upfront triple combination therapy. While TRITON trial did not show significant improvement in pulmonary vascular resistance with upfront triple combination therapy, there was a reduction in the risk of disease progression, and exploratory analysis suggested a trend toward improved long-term outcomes. 17 More recently, a post hoc analysis of the pooled data for newly diagnosed (⩽6 months) PAH patients (44% on background dual combination therapy) from GRIPHON and TRITON trials showed a reduction in disease progression by 52% and risk of death, hazard ratio (HR) 0.70 (95% CI: 0.46–1.10) compared to control group with early addition of selexipag. 18
Current guidelines support add-on sequential triple combination therapy, particularly prostacyclin receptor agonist (selexipag) for intermediate-low-risk individuals based on the GRIPHON trial and parenteral prostacyclin for intermediate-high or high-risk individuals.7,19 –21 However, data on other forms of nonparenteral prostanoids are scarce, and current recommendations are consensus or expert opinion based limited to individual case-based approach (Grade IIb or ungraded).20,21 The FREEDOM C2 study included 65 patients who received oral treprostinil as a third add-on drug on a background regimen of ERA and PDE-5 inhibitor and found no significant improvement in exercise capacity at 16 weeks. 22 Post hoc analysis of TRIUMPH and BEAT demonstrated an improvement in REVEAL 2.0 risk stratum and score with the addition of inhaled treprostinil and included 15% of patients on background dual combination therapy. 23
Olsson et al. 24 studied 126 patients on background combination therapy, with 79% patients in the intermediate-risk category and 19% in the high-risk category who were initiated on add-on intravenous treprostinil therapy. They found that up to 19% of patients achieved low-risk status and had a 5-year transplant-free survival of greater than 90%. 24 Smaller retrospective studies have also demonstrated stabilization and improvement in clinical parameters with add-on selexipag.25 –27
In our cohort, we deemed necessary to add a third drug in 25% of patients, based on clinical progression or lack of improvement. Our study highlights a significant improvement in risk status and clinical and hemodynamic parameters even at a median time from diagnosis to the addition of the third drug of 120 weeks. The present study also demonstrated achievement or maintenance of low-risk status in 61.8% of patients, which resulted in significant improvement in long-term survival seen on Kaplan–Meier survival curves (Figure 7). In clinical practice, choice of combination regimen may depend on the adverse effect profile, etiology and severity of PAH, medication interactions, and insurance coverage. While we were able to demonstrate clinical and hemodynamic improvement with the addition of a third drug, it is notable that not all patients achieved recommended therapeutic goal. Up to 22% (12/55) of patients were still in intermediate-risk category, whereas 16% (9/55) of them were at high-risk of progression and death. Similarly, the right ventricle was visually hypokinetic in 45% (25/55), and TAPSE was low in 25% (14/55) of the patients. Finally, the mean 6-min walk distance achieved was only 317 (±107) m, and median PVR at the time of the first RHC after addition of a third drug was 5.77 (4.50, 7.83) Wood Units. These findings underscore the need for rigorous follow-up and therapeutic optimization including maximization of therapy to parenteral prostacyclins. It is also important to identify predictors of risk reduction upon introduction of a third drug, since it may assist in decision-making on escalating pharmacologic management versus early transplant referral. Patients with pericardial effusion and elevated estimated right atrial pressure on echocardiogram showed lower odds of risk reduction. These findings are hypothesis-generating and consistent with current literature as both parameters are important prognostic indicators and signify an advanced disease state with pulmonary vascular and right ventricular remodeling. 28 Similar to prior studies, our cohort predominantly comprises women (85%). 29 It is well-known that women have increased susceptibility to the development of PAH. However, the treatment response and survival are also better. 29 Our study also identified the female gender as a strong predictor of successful risk reduction.
Interestingly, our study found patients of Hispanic ethnicity to have lower odds of successful risk reduction, but the reason for this finding is unclear. On the contrary, most South American registries demonstrate slightly higher survival rates when compared to the United States, but this may be due to the under-representation of Hispanic population and older age of enrolled patients in US registries. 30 Karnes et al. 31 found reproducible survival benefit in Hispanic patient cohorts in multiple clinical settings based on two US registries. Medrek et al. 32 did not find race/ethnicity as a significant predictor of mortality in REVEAL registry. Al-Naamani et al. 33 have previously demonstrated an increased burden and severity of PAH-associated disorders such as HIV and CTD in various racial and ethnic groups. However, most registries lack diversity (e.g., 12.3% Black and 2.3% Hispanic patients in the National Institute of Health registry).34,35 Under-representation of the non-White cohort is also reflected in most clinical trials with 85% enrolled White patients in earlier studies, which decreased overtime to 70%. 36 In our cohort, we found that addition of a third drug in non-White patients also led to significant improvement in risk status, and functional, echocardiographic, and hemodynamic parameters along with improved survival in patients with successful risk reduction (Figure 8). Further studies are needed to evaluate the true impact of racial/ethnic backgrounds on outcomes in PAH.
We also identified that most patients tolerated triple combination therapy well, but there was a significant increase in adverse effects, predominantly gastrointestinal (nausea, vomiting, or diarrhea) which was expected as they are known effects of prostacyclin-based therapies. However, the sample size was small to assess the differences between each regimen.
Strengths and limitations
The present study has several strengths and limitations. First, we highlight an essential aspect of treatment strategy in PAH patients in a diverse population with long-term clinical and survival impact. This study supports the view of considering risk reduction as an endpoint in future drug trials and prospectively assess the impact of sequential triple sequential therapy on survival.
The major limitation of this study is the small sample size, retrospective and single-center design, and limiting generalizability. In addition, patients initiated on triple therapy but had not had a follow-up assessment were excluded from the study, thus further limiting the sample size. We also did not consider the exact dosing of each medication as it is well-known that certain drugs have a higher therapeutic impact. The study aimed to assess an overall interval change in clinical parameters with a third drug rather than compare various formularies or combinations. Multicenter, prospective studies are needed to identify the best triple combination regimen for PAH according to various etiologies.
Conclusion
Our study confirms a sustained and long-term clinical and survival benefit with add-on sequential triple combination therapy in a racially diverse population. Parenteral prostacyclin is needed for WHO FC IV or patients who demonstrate rapid clinical decline. We also demonstrate that female gender is an independent predictor of successful risk reduction. Presence of pericardial effusion and elevated right atrial pressure on initial evaluation may suggest advanced pulmonary vascular remodeling and predict lower odds of successful risk reduction. Since not all patients achieve therapeutic goal with the sequential triple drug regimen, there is a need for continuous and rigorous monitoring and, as needed, up-titration of therapy and/or transplant referral. In our cohort, patients of Hispanic ethnicity have lower odds of risk reduction, and these findings need to be explored in larger studies. Multicenter, prospective studies are needed to confirm the findings of this study.
Acknowledgments
None.
Footnotes
ORCID iD: Himanshu Deshwal  https://orcid.org/0000-0003-2086-1281
https://orcid.org/0000-0003-2086-1281
Contributor Information
Himanshu Deshwal, Pulmonary Hypertension Clinic (Pulmonology), Division of Pulmonary, Sleep, and Critical Care Medicine, West Virginia University School of Medicine, 1 Medical Center Drive, Morgantown, WV 26505, USA.
Tatiana Weinstein, Pulmonary Hypertension Program, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, New York University Grossman School of Medicine, NYU Langone Health, New York, NY, USA.
Rachel Salyer, Department of Medicine, West Virginia Clinical and Translational Science Institute, West Virginia University, Morgantown, WV, USA.
Jesse Thompson, Department of Medicine, West Virginia Clinical and Translational Science Institute, West Virginia University, Morgantown, WV, USA.
Frank Cefali, Pulmonary Hypertension Program, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, New York University Grossman School of Medicine, NYU Langone Health, New York, NY, USA.
Rebecca Fenton, Pulmonary Hypertension Program, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, New York University Grossman School of Medicine, NYU Langone Health, New York, NY, USA.
Eric Bondarsky, Pulmonary Hypertension Program, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, New York University Grossman School of Medicine, NYU Langone Health, New York, NY, USA.
Roxana Sulica, Pulmonary Hypertension Program, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, New York University Grossman School of Medicine, NYU Langone Health, New York, NY, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Himanshu Deshwal: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Validation; Visualization; Writing – original draft; Writing – review & editing.
Tatiana Weinstein: Data curation; Writing – original draft; Writing – review & editing.
Rachel Salyer: Methodology; Writing – review & editing.
Jesse Thompson: Methodology; Writing – review & editing.
Frank Cefali: Methodology; Writing – review & editing.
Rebecca Fenton: Writing – review & editing.
Eric Bondarsky: Data curation; Methodology; Writing – review & editing.
Roxana Sulica: Conceptualization; Data curation; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
HD, TW, JT, FC, RS (Salyer), and RF have no conflicts of interest or financial conflicts to disclose. RS (Sulica) has received research support from Bayer, United Therapeutics, Acceleron, Bellerophon, Gossamer, Enzyvant, Aerovate, and Merck and served on advisory boards for Actelion, Janssen, Enzyvant, Merck, Bayer, Gossamer, and United Therapeutics. Preliminary results of this study were presented as a thematic poster discussion at the American Thoracic Society International Conference 2022, held in San Francisco, USA, on 16 May 2022.
Availability of data and materials: Not applicable.
References
- 1. Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL registry. Chest 2012; 142: 448–456. [DOI] [PubMed] [Google Scholar]
- 2. Thenappan T, Shah SJ, Rich S, et al. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J 2010; 35: 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang KY, Duval S, Badesch DB, et al. Mortality in pulmonary arterial hypertension in the modern era: early insights from the pulmonary hypertension association registry. J Am Heart Assoc 2022; 11: e024969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323–337. [DOI] [PubMed] [Google Scholar]
- 5. Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700889. [DOI] [PubMed] [Google Scholar]
- 6. Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. New Engl J Med 2015; 373: 834–844. [DOI] [PubMed] [Google Scholar]
- 7. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS). Endorsed by the international society for heart and lung transplantation (ISHLT) and the European reference network on rare respiratory diseases (ERN-LUNG). Eur Heart J 2022; 43: 3618–3731.36017548 [Google Scholar]
- 8. Weatherald J, Boucly A, Sahay S, et al. The low-risk profile in pulmonary arterial hypertension. Time for a paradigm shift to goal-oriented clinical trial endpoints? Am J Respir Crit Care Med 2018; 197: 860–868. [DOI] [PubMed] [Google Scholar]
- 9. Boucly A, Weatherald J, Savale L, et al. External validation of a refined 4-strata risk assessment score from the French pulmonary hypertension registry. Eur Respir J 2021; 59: 2102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoeper MM, Pausch C, Olsson KM, et al. COMPERA 2.0: a refined four-stratum risk assessment model for pulmonary arterial hypertension. Eur Respir J 2022; 60: 2102311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sulica R, Sangli S, Chakravarti A, et al. Clinical and hemodynamic benefit of macitentan and riociguat upfront combination in patients with pulmonary arterial hypertension. Pulm Circ 2019; 9: 2045894019826944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Badagliacca R, D’Alto M, Ghio S, et al. Risk reduction and hemodynamics with initial combination therapy in pulmonary arterial hypertension. Am J Respir Crit Care Med 2020; 203: 484–492. [DOI] [PubMed] [Google Scholar]
- 13. Kylhammar D, Hjalmarsson C, Hesselstrand R, et al. Predicting mortality during long-term follow-up in pulmonary arterial hypertension. ERJ Open Res 2021; 7: 00837–02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van de Veerdonk MC, Marcus JT, Westerhof N, et al. Signs of right ventricular deterioration in clinically stable patients with pulmonary arterial hypertension. Chest 2015; 147: 1063–1071. [DOI] [PubMed] [Google Scholar]
- 15. D’Alto M, Badagliacca R, Argiento P, et al. Risk reduction and right heart reverse remodeling by upfront triple combination therapy in pulmonary arterial hypertension. Chest 2020; 157: 376–383. [DOI] [PubMed] [Google Scholar]
- 16. Boucly A, Savale L, Jaïs X, et al. Association between initial treatment strategy and long-term survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 2021; 204: 842–854. [DOI] [PubMed] [Google Scholar]
- 17. Chin KM, Sitbon O, Doelberg M, et al. Three- versus two-drug therapy for patients with newly diagnosed pulmonary arterial hypertension. J Am Coll Cardiol 2021; 78: 1393–1403. [DOI] [PubMed] [Google Scholar]
- 18. Coghlan JG, Gaine S, Channick R, et al. Early selexipag initiation and long-term outcomes: insights from RCTs in PAH. ERJ Open Res 2022; 9: 00456–02022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. New Engl J Med 2015; 373: 2522–2533. [DOI] [PubMed] [Google Scholar]
- 20. Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): endorsed by: association for European pediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 21. Klinger JR, Elliott CG, Levine DJ, et al. Therapy for pulmonary arterial hypertension in adults: update of the CHEST guideline and expert panel report. Chest 2019; 155: 565–586. [DOI] [PubMed] [Google Scholar]
- 22. Tapson VF, Jing ZC, Xu KF, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest 2013; 144: 952–958. [DOI] [PubMed] [Google Scholar]
- 23. Tonelli AR, Sahay S, Gordon KW, et al. Impact of inhaled treprostinil on risk stratification with noninvasive parameters: a post hoc analysis of the TRIUMPH and BEAT studies. Pulm Circ 2020; 10: 2045894020977025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olsson KM, Richter MJ, Kamp JC, et al. Intravenous treprostinil as an add-on therapy in patients with pulmonary arterial hypertension. J Heart Lung Transplant 2019; 38: 748–756. [DOI] [PubMed] [Google Scholar]
- 25. Momoi M, Hiraide T, Shinya Y, et al. Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension. Ther Adv Respir Dis 2021; 15: 1753466621995048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berlier C, Schwarz EI, Saxer S, et al. Real-life experience with selexipag as an add-on therapy to oral combination therapy in patients with pulmonary arterial or distal chronic thromboembolic pulmonary hypertension: a retrospective analysis. Lung 2019; 197: 353–360. [DOI] [PubMed] [Google Scholar]
- 27. Taçoy G, Çengel A, Alsancak Y, et al. Combination therapy in pulmonary arterial hypertension: single centre long-term experience. West Indian Med J 2015; 65: 46–51. [DOI] [PubMed] [Google Scholar]
- 28. Grapsa J, Nunes MCP, Tan TC, et al. Echocardiographic and hemodynamic predictors of survival in precapillary pulmonary hypertension. Circ Cardiovasc Imaging 2015; 8: e002107. [DOI] [PubMed] [Google Scholar]
- 29. Cheron C, McBride SA, Antigny F, et al. Sex and gender in pulmonary arterial hypertension. Eur Respir Rev 2021; 30: 200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Valverde AB, Soares JM, Viana KP, et al. Pulmonary arterial hypertension in Latin America: epidemiological data from local studies. BMC Pulm Med 2018; 18: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karnes JH, Wiener HW, Schwantes-An TH, et al. Genetic admixture and survival in diverse populations with pulmonary arterial hypertension. Am J Respir Crit Care Med 2020; 201: 1407–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Medrek S, Sahay S, Zhao C, et al. Impact of race on survival in pulmonary arterial hypertension: results from the REVEAL registry. J Heart Lung Transplant 2020; 39: 321–330. [DOI] [PubMed] [Google Scholar]
- 33. Al-Naamani N, Paulus JK, Roberts KE, et al. Racial and ethnic differences in pulmonary arterial hypertension. Pulm Circ 2017; 7: 793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 35. Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US contemporary registries. Chest 2011; 139: 128–137. [DOI] [PubMed] [Google Scholar]
- 36. Min J, Appleby DH, McClelland RL, et al. Secular and regional trends among pulmonary arterial hypertension clinical trial participants. Ann Am Thorac Soc 2021; 19: 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]