Figure 1. Diversity of Glutamatergic PNs and models of PN diversification in the developing neocortex.
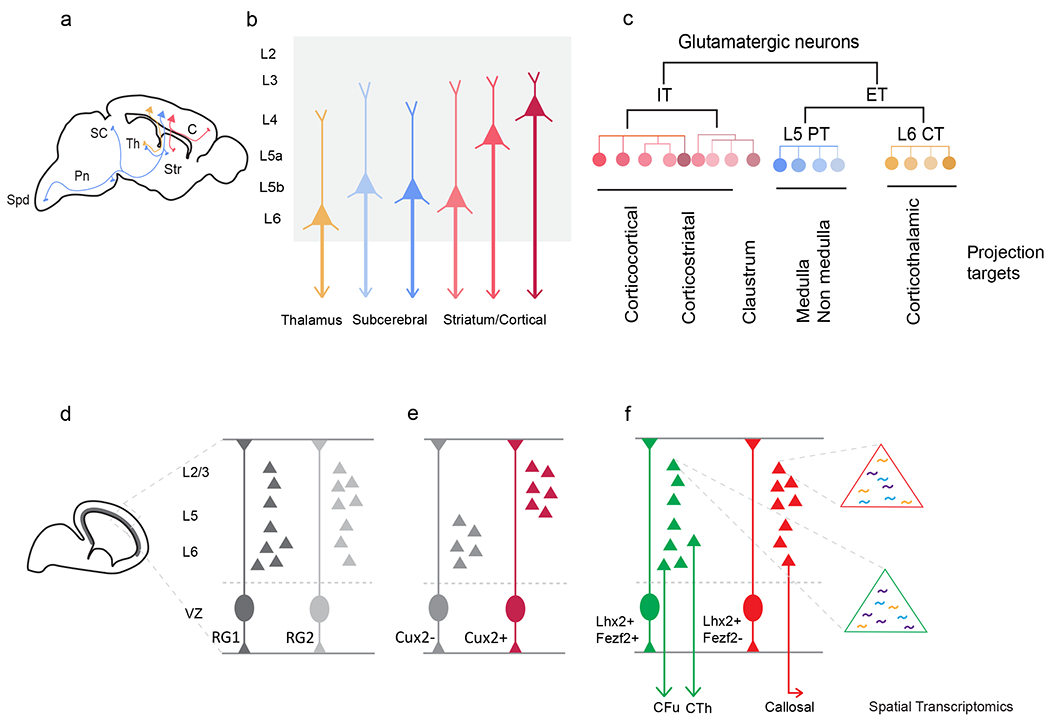
a. Sagittal schematic showing major PN classes and their projection targets to intratelencephalic regions (IT, Red), subcerebral targets (PT, Blue), and thalamus (CT, Yellow). C, cortex; SC, superior colliculus; Str, striatum; Th, thalamus; Pn, pons; Spd, spinal cord. b. Schematic representation of major PN types based on their laminar location and projection targets. c. Hierarchical organization of PN based on transcriptomic profiles and projection targets in the neocortex. ET, Extratelencephalic; PT, Pyramidal tract; CT, Corticothalamic. d. Sagittal schematic of embryonic mouse brain at E12.5 showing ventricular zone (Gray) where RG progenitors reside and differentiate. Model of uniform multipotent progenitors, where a single lineage of RGs generates all types of PNs and the competence of RG to generate specific types becomes progressively restricted over the course of development. E. Model of fate-restricted progenitors, where distinct lineages of RGs co-exist and are specified to generated different types of PNs. Cux2− RGs are competent to generate layer 5-6 corticofugal PN while Cux2+ RGs generate L2-4 corticocortical PN. F. Fate-mapping based on TF (Fezf2 and Lhx2)-defined RGs derived PN population suggests the presence of fate-restricted progenitors [Ref.45]. PNs generated from RGLhx2+Fezf2+ are extratelencephalic (Green) while RGLhx2+Fezf2− derived PN project to intratelencephalic regions (Red). Schematic representation of the use of spatial transcriptomics to reveal the molecular identity of fate-mapped PNs from TF-defined progenitors (ET, Green; IT, Red). CFu, Corticofugal; CTh, Corticothalamic.
