Abstract
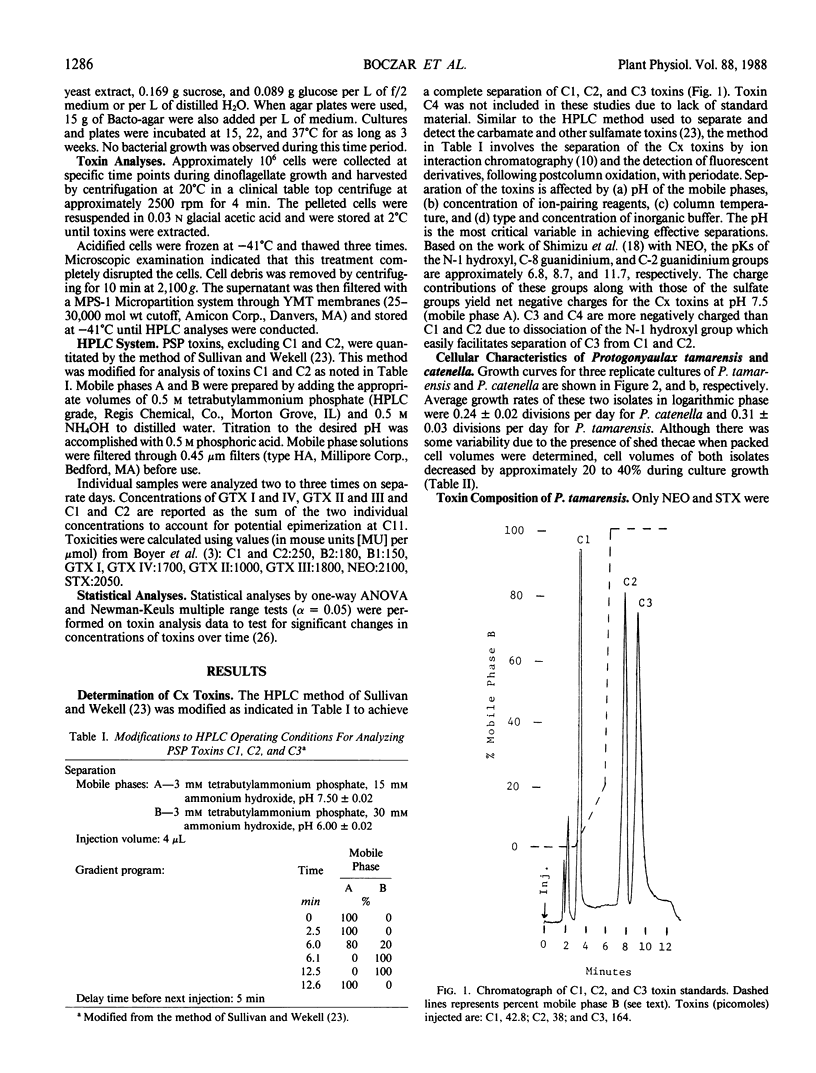
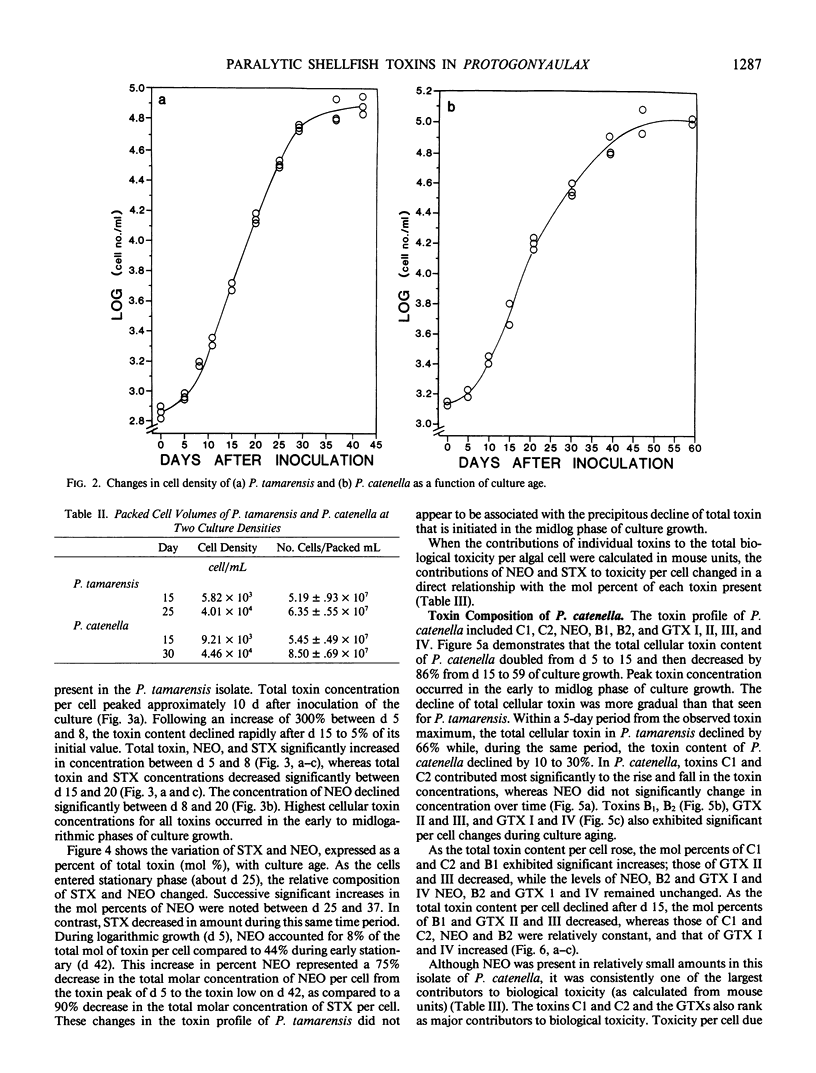
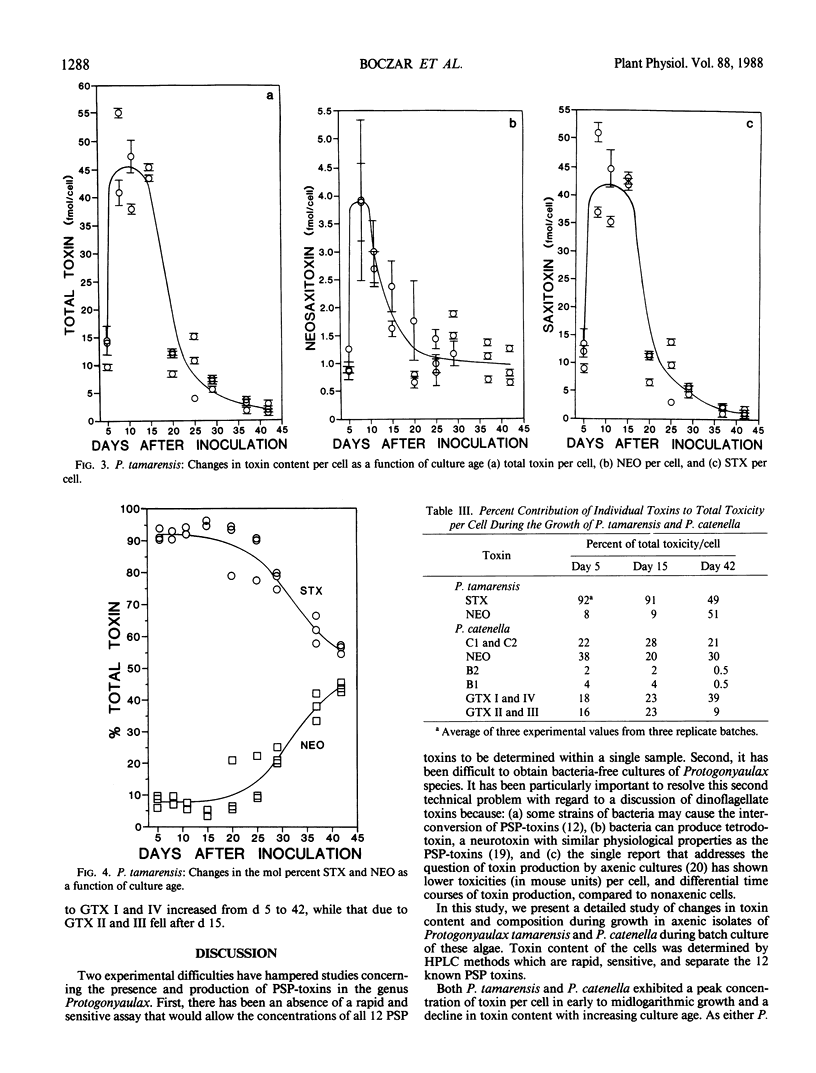
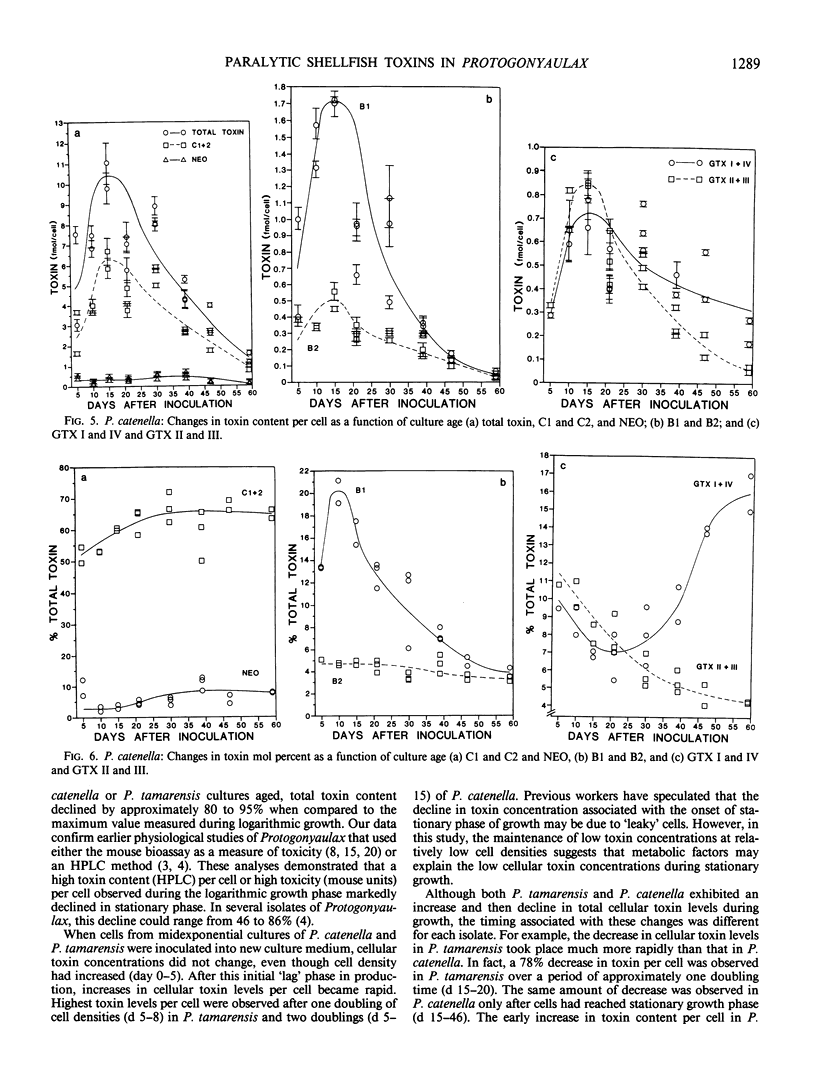
Paralytic shellfish toxin concentrations were measured and individual toxin profiles were monitored in axenic batch cultures of Protogonyaulax tamarensis and Protogonyaulax catenella. High pressure liquid chromatographic methods were used that allowed the separation of all 12 known paralytic shellfish poisons, including toxins C1, C2, and C3, from a single sample. In isolates of both Protogonyaulax species, total toxin levels were relatively low after inoculation, increased rapidly in early to mid-exponential growth to a value 100 to 300% of that at the initial time point, then decreased by 86 to 95% as the culture aged. Although the concentrations of individual toxins per cell followed the same general pattern as that seen for total moles of toxin per cell, variability in toxin profile with culture age was observed. In P. tamarensis, the mole percent of neosaxitoxin increased substantially from 8 to 44% as total toxin levels per cell decreased. A concomitant decrease in the mole percent of saxitoxin with culture age was noted. Although not as precipitous, changes in the mole percent of specific toxins from P. catenella were also observed. The mole percent of gonyautoxins I and IV increased, while that of gonyautoxins II and III decreased. These data suggest that the toxin profile in isolates of Protogonyaulax can change, sometimes significantly, with changing environmental variables.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GUILLARD R. R., RYTHER J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962 Apr;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Jackim E., Gentile J. Toxins of a blue-green alga: similarity to saxitoxin. Science. 1968 Nov 22;162(3856):915–916. doi: 10.1126/science.162.3856.915. [DOI] [PubMed] [Google Scholar]
- Simidu U., Noguchi T., Hwang D. F., Shida Y., Hashimoto K. Marine bacteria which produce tetrodotoxin. Appl Environ Microbiol. 1987 Jul;53(7):1714–1715. doi: 10.1128/aem.53.7.1714-1715.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. J., Iwaoka W. T. High pressure liquid chromatographic determination of toxins associated with paralytic shellfish poisoning. J Assoc Off Anal Chem. 1983 Mar;66(2):297–303. [PubMed] [Google Scholar]
- Watanabe N., Arimura A., Kobayashi M., Oshima M. Anti-NADase values in rheumatic fever and valvular disease. Jpn Circ J. 1984 Dec;48(12):1343–1344. doi: 10.1253/jcj.48.1343. [DOI] [PubMed] [Google Scholar]


