Abstract
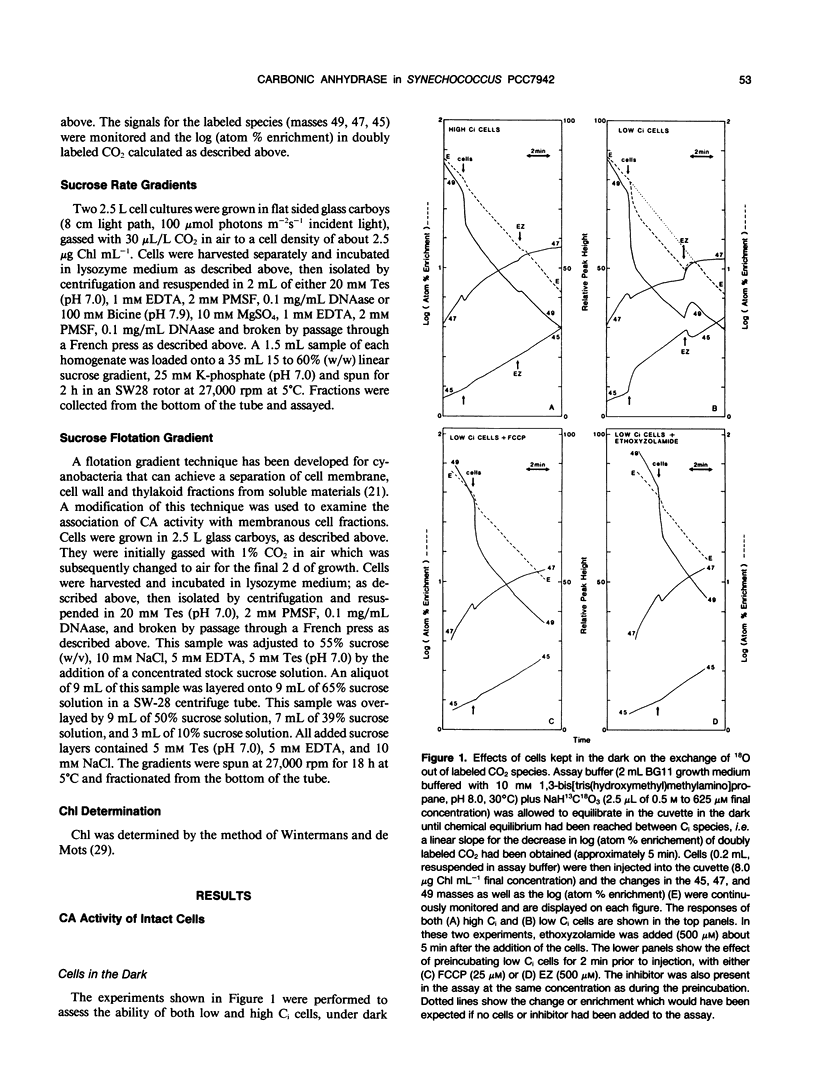
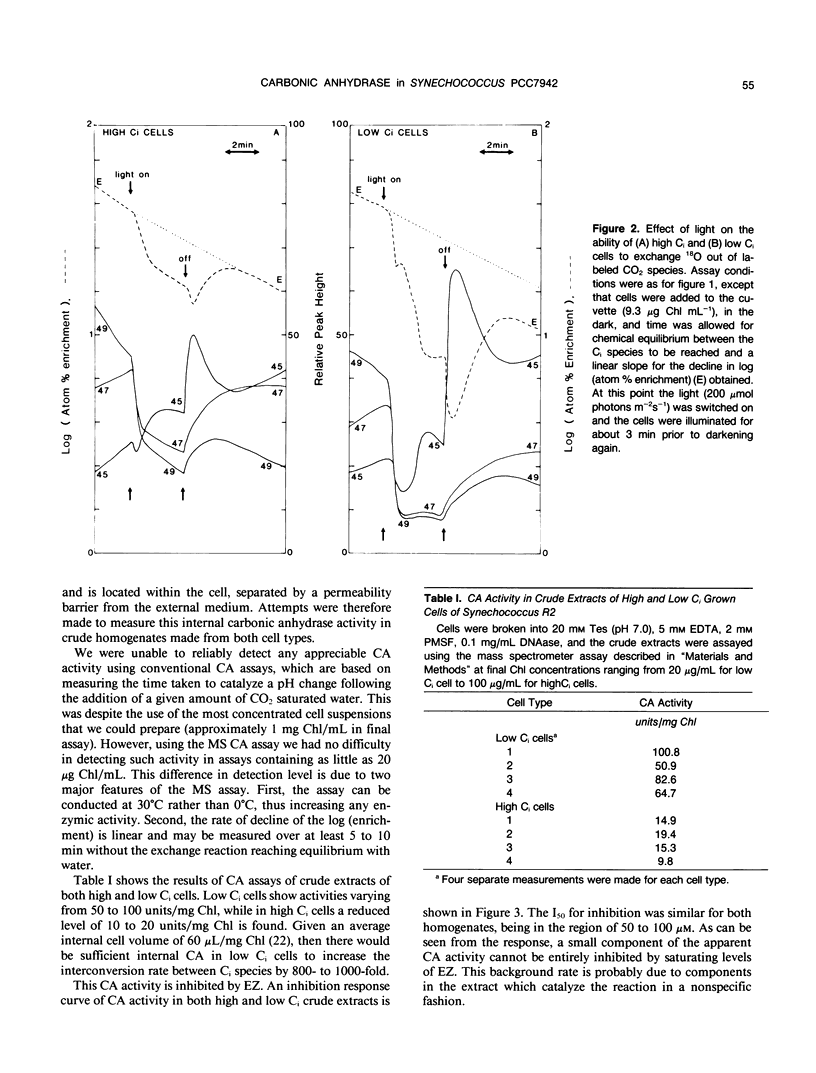
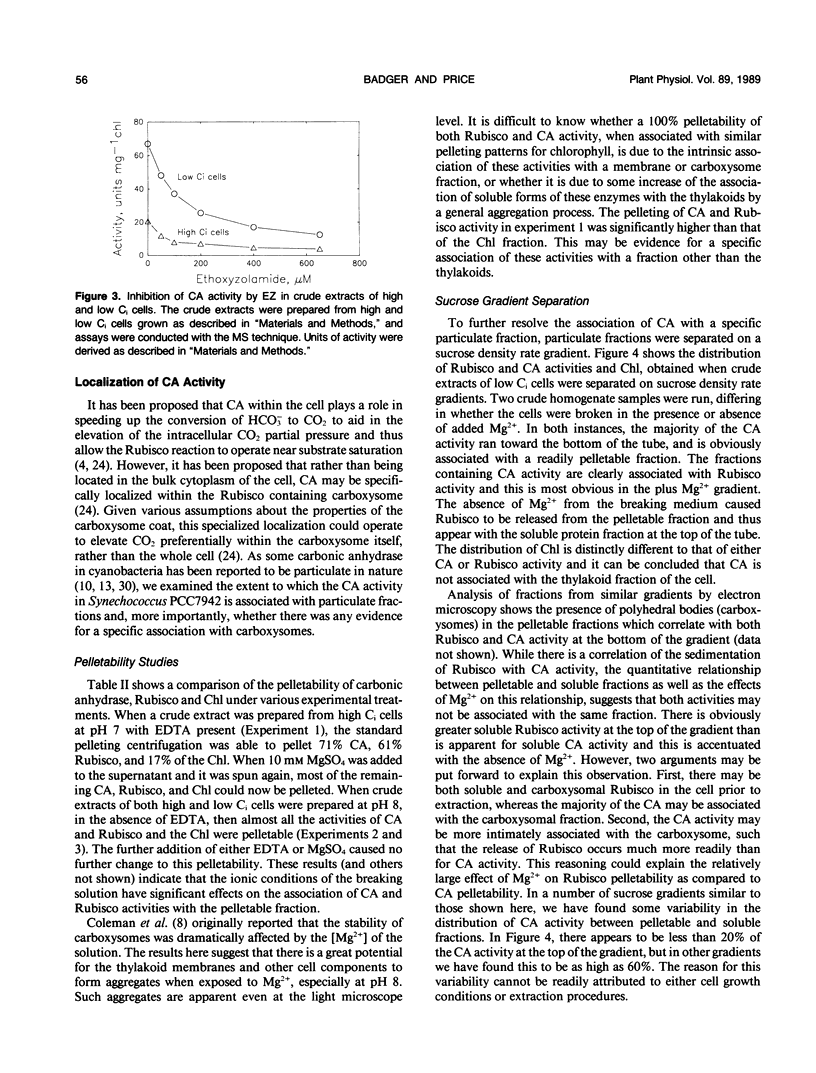
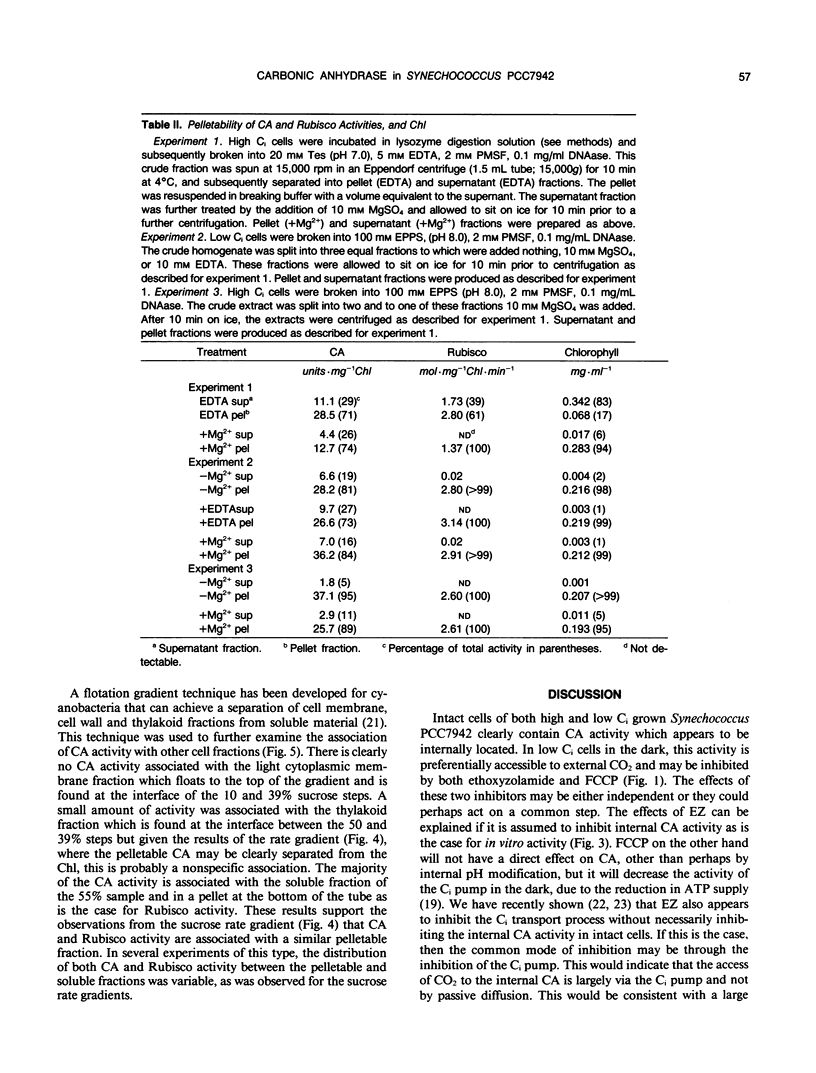
Intact cells and crude homogenates of high (1% CO2) and low dissolved inorganic carbon (Ci) (30-50 microliters per liter of CO2) grown Synechococcus PCC7942 have carbonic anhydrase (CA)-like activity, which enables them to catalyze the exchange of 18O from CO2 to H2O. This activity was studied using a mass spectrometer coupled to a cuvette with a membrane inlet system. Intact high and low Ci cells were found to contain CA activity, separated from the medium by a membrane which is preferentially permeable to CO2. This activity is most apparent in the light, where 18O-labeled CO2 species are being taken up by the cells but the effluxing CO2 has lost most of its label to water. In the dark, low Ci cells catalyze the depletion of the 18O enrichment of CO2 and this activity is inhibited by both ethoxyzolamide and 2-(trifluoromethoxy)carbonyl cyanide. This may occur via a common inhibition of the Ci pump and the Ci pump is proposed as a potential site for the exchange of 18O. CA activity was measurable in homogenates of both cell types but was 5- to 10-fold higher in low Ci cells. This was inhibited by ethoxyzolamide with an I50 of 50 to 100 micromolar in both low and high Ci cells. A large proportion of the internal CA activity appears to be pelletable in nature. This pelletability is increased by the presence of Mg2+ in a manner similar to that of ribulose bisphosphate carboxylase-oxygenase activity and chlorophyll (thylakoids) and may be the result of nonspecific aggregation. Separation of crude homogenates on sucrose gradients is consistent with the notion that CA and ribulose bisphosphate carboxylase-oxygenase activity may be associated with the same pelletable fraction. However, we cannot unequivocally establish that CA is located within the carboxysome. The sucrose gradients show the presence of separate soluble and pelletable CA activity. This may be due to the presence of separate forms of the enzyme or may arise from the same pelletable association which is unstable during extraction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Andrews T. J. Photosynthesis and Inorganic Carbon Usage by the Marine Cyanobacterium, Synechococcus sp. Plant Physiol. 1982 Aug;70(2):517–523. doi: 10.1104/pp.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Bassett M., Comins H. N. A Model for HCO(3) Accumulation and Photosynthesis in the Cyanobacterium Synechococcus sp: Theoretical Predictions and Experimental Observations. Plant Physiol. 1985 Feb;77(2):465–471. doi: 10.1104/pp.77.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank R. T., Badger M. R., Osmond C. B. Photosynthetic oxygen exchange in isolated cells and chloroplasts of c(3) plants. Plant Physiol. 1982 Oct;70(4):927–931. doi: 10.1104/pp.70.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Schwarz R., Friedberg D., Kaplan A. High CO(2) Requiring Mutant of Anacystis nidulans R(2). Plant Physiol. 1986 Oct;82(2):610–612. doi: 10.1104/pp.82.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo W. P., Williams T. G., Birch D. G., Turpin D. H. Photosynthetic Adaptation by Synechococcus leopoliensis in Response to Exogenous Dissolved Inorganic Carbon. Plant Physiol. 1986 Apr;80(4):1038–1040. doi: 10.1104/pp.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Husic H. D., Tolbert N. E. Effect of Carbonic Anhydrase Inhibitors on Inorganic Carbon Accumulation by Chlamydomonas reinhardtii. Plant Physiol. 1985 Sep;79(1):177–183. doi: 10.1104/pp.79.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Kitayama M., Togasaki R. K., Tolbert N. E. Evidence for Inorganic Carbon Transport by Intact Chloroplasts of Chlamydomonas reinhardtii. Plant Physiol. 1987 Mar;83(3):460–463. doi: 10.1104/pp.83.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kaneda T., Omata T. A Mutant of Synechococcus PCC7942 Incapable of Adapting to Low CO(2) Concentration. Plant Physiol. 1987 Jul;84(3):711–715. doi: 10.1104/pp.84.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T., Ogawa T. Biosynthesis of a 42-kD Polypeptide in the Cytoplasmic Membrane of the Cyanobacterium Anacystis nidulans Strain R2 during Adaptation to Low CO(2) Concentration. Plant Physiol. 1986 Feb;80(2):525–530. doi: 10.1104/pp.80.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. N. Carbonic anhydrase: oxygen-18 exchange catalyzed by an enzyme with rate-contributing proton-transfer steps. Methods Enzymol. 1982;87:732–752. doi: 10.1016/s0076-6879(82)87037-7. [DOI] [PubMed] [Google Scholar]
- Tu C. K., Acevedo-Duncan M., Wynns G. C., Silverman D. N. Oxygen-18 Exchange as a Measure of Accessibility of CO(2) and HCO(3) to Carbonic Anhydrase in Chlorella vulgaris (UTEX 263). Plant Physiol. 1986 Apr;80(4):997–1001. doi: 10.1104/pp.80.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C., Spiller H., Wynns G. C., Silverman D. N. Carbonic Anhydrase and the Uptake of Inorganic Carbon by Synechococcus sp. (UTEX-2380). Plant Physiol. 1987 Sep;85(1):72–77. doi: 10.1104/pp.85.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Wolpert T. J., Macko V., Acklin W., Arigoni D. Molecular Features Affecting the Biological Activity of the Host-Selective Toxins from Cochliobolus victoriae. Plant Physiol. 1988 Sep;88(1):37–41. doi: 10.1104/pp.88.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]


